The basal ganglia is necessary for learning spectral, but not temporal, features of birdsong
- PMID: 24075977
- PMCID: PMC3929499
- DOI: 10.1016/j.neuron.2013.07.049
The basal ganglia is necessary for learning spectral, but not temporal, features of birdsong
Abstract
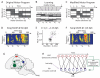
Executing a motor skill requires the brain to control which muscles to activate at what times. How these aspects of control-motor implementation and timing-are acquired, and whether the learning processes underlying them differ, is not well understood. To address this, we used a reinforcement learning paradigm to independently manipulate both spectral and temporal features of birdsong, a complex learned motor sequence, while recording and perturbing activity in underlying circuits. Our results uncovered a striking dissociation in how neural circuits underlie learning in the two domains. The basal ganglia was required for modifying spectral, but not temporal, structure. This functional dissociation extended to the descending motor pathway, where recordings from a premotor cortex analog nucleus reflected changes to temporal, but not spectral, structure. Our results reveal a strategy in which the nervous system employs different and largely independent circuits to learn distinct aspects of a motor skill.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



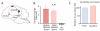
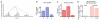

References
-
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J. Chem. Neuroanat. 2000;18:117–133. - PubMed
-
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
