Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention
- PMID: 24076049
- PMCID: PMC3805703
- DOI: 10.1016/j.immuni.2013.08.019
Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention
Abstract
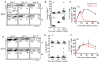
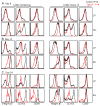
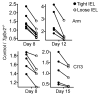
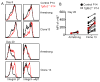
Tissue-resident memory T (Trm) cells represent a population of memory CD8⁺ T cells that can act as first responders to local infection. The mechanisms regulating the formation and maintenance of intestinal Trm cells remain elusive. Here we showed that transforming growth factor-β (TGF-β) controlled both stages of gut Trm cell differentiation through different mechanisms. During the formation phase of Trm cells, TGF-β signaling inhibited the migration of effector CD8⁺ T cells from the spleen to the gut by dampening the expression of integrin α4β7. During the maintenance phase, TGF-β was required for the retention of intestinal Trm cells at least in part through the induction of integrins αEβ7 and α1, as well as CD69. Thus, the cytokine acts to control cytotoxic T cell differentiation in lymphoid and peripheral organs.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. - PubMed
-
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. - PubMed
-
- Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

