Mu opioids and their receptors: evolution of a concept
- PMID: 24076545
- PMCID: PMC3799236
- DOI: 10.1124/pr.112.007138
Mu opioids and their receptors: evolution of a concept
Abstract
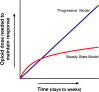
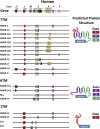
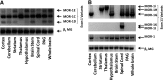
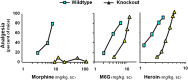
Opiates are among the oldest medications available to manage a number of medical problems. Although pain is the current focus, early use initially focused upon the treatment of dysentery. Opium contains high concentrations of both morphine and codeine, along with thebaine, which is used in the synthesis of a number of semisynthetic opioid analgesics. Thus, it is not surprising that new agents were initially based upon the morphine scaffold. The concept of multiple opioid receptors was first suggested almost 50 years ago (Martin, 1967), opening the possibility of new classes of drugs, but the morphine-like agents have remained the mainstay in the medical management of pain. Termed mu, our understanding of these morphine-like agents and their receptors has undergone an evolution in thinking over the past 35 years. Early pharmacological studies identified three major classes of receptors, helped by the discovery of endogenous opioid peptides and receptor subtypes-primarily through the synthesis of novel agents. These chemical biologic approaches were then eclipsed by the molecular biology revolution, which now reveals a complexity of the morphine-like agents and their receptors that had not been previously appreciated.
Figures



















References
-
- Abbadie C, Gultekin SH, Pasternak GW. (2000a) Immunohistochemical localization of the carboxy terminus of the novel mu opioid receptor splice variant MOR-1C within the human spinal cord. Neuroreport 11:1953–1957 - PubMed
-
- Abbadie C, Pan Y-X, Drake CT, Pasternak GW. (2000b) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100:141–153 - PubMed
-
- Abbadie C, Pan Y-X, Pasternak GW. (2000c) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol 419:244–256 - PubMed
-
- Abbadie C, Pan Y-X, Pasternak GW. (2004) Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in mouse brain. Neuroscience 127:419–430 - PubMed
-
- Abbadie C, Pasternak GW. (2001) Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport 12:3069–3072 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA013997/DA/NIDA NIH HHS/United States
- R56 DA002615/DA/NIDA NIH HHS/United States
- DA00220/DA/NIDA NIH HHS/United States
- R01 DA006241/DA/NIDA NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R21 DA029244/DA/NIDA NIH HHS/United States
- R37 DA007242/DA/NIDA NIH HHS/United States
- R01 DA007242/DA/NIDA NIH HHS/United States
- DA06241/DA/NIDA NIH HHS/United States
- R01 DA002615/DA/NIDA NIH HHS/United States
- DA029244/DA/NIDA NIH HHS/United States
- T32 DA007242/DA/NIDA NIH HHS/United States
- K05 DA000220/DA/NIDA NIH HHS/United States
- R01 DA013997/DA/NIDA NIH HHS/United States
- DA02615/DA/NIDA NIH HHS/United States
- DA07242/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

