Self-assembly of carbon nanotubes and antibodies on tumours for targeted amplified delivery
- PMID: 24077028
- PMCID: PMC3798027
- DOI: 10.1038/nnano.2013.190
Self-assembly of carbon nanotubes and antibodies on tumours for targeted amplified delivery
Abstract
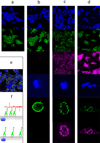
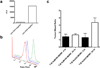
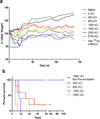
Single-walled carbon nanotubes (SWNTs) can deliver imaging agents or drugs to tumours and offer significant advantages over approaches based on antibodies or other nanomaterials. In particular, the nanotubes can carry a substantial amount of cargo (100 times more than a monoclonal antibody), but can still be rapidly eliminated from the circulation by renal filtration, like a small molecule, due to their high aspect ratio. Here we show that SWNTs can target tumours in a two-step approach in which nanotubes modified with morpholino oligonucleotide sequences bind to cancer cells that have been pretargeted with antibodies modified with oligonucleotide strands complementary to those on the nanotubes. The nanotubes can carry fluorophores or radioisotopes, and are shown to selectively bind to cancer cells in vitro and in tumour-bearing xenografted mice. The binding process is also found to lead to antigen capping and internalization of the antibody-nanotube complexes. The nanotube conjugates were labelled with both alpha-particle and gamma-ray emitting isotopes, at high specific activities. Conjugates labelled with alpha-particle-generating (225)Ac were found to clear rapidly, thus mitigating radioisotope toxicity, and were shown to be therapeutically effective in vivo.
Figures

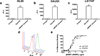
Comment in
-
New technology: nanotechnology targets cancer cells.Nat Rev Clin Oncol. 2013 Dec;10(12):667. doi: 10.1038/nrclinonc.2013.186. Epub 2013 Oct 15. Nat Rev Clin Oncol. 2013. PMID: 24129354 No abstract available.
References
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. - PubMed
-
- Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nature Nanotech. 2009;4:627–633. - PubMed
-
- Liu Z, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nature Nanotech. 2007;2:47–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

