Isolation and characterization of squamous cell carcinoma-derived stem-like cells: role in tumor formation
- PMID: 24077125
- PMCID: PMC3821572
- DOI: 10.3390/ijms141019540
Isolation and characterization of squamous cell carcinoma-derived stem-like cells: role in tumor formation
Abstract
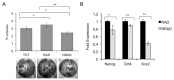
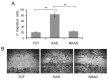
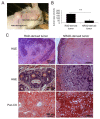
In human epidermis, keratinocyte stem cells (KSC) are characterized by high levels of β1-integrin, resulting in the rapid adhesion to type IV collagen. Since epithelial tumors originate from KSC, we evaluated the features of rapidly adhering (RAD) keratinocytes derived from primary human squamous cell carcinoma of the skin (cSCC). RAD cells expressed higher levels of survivin, a KSC marker, as compared to non-rapidly adhering (NRAD) cells. Moreover, RAD cells proliferated to a greater extent and were more efficient in forming colonies than NRAD cells. RAD cells also migrated significantly better than NRAD cells. When seeded in a silicone chamber and grafted onto the back skin of NOD SCID mice, RAD cells formed tumors 2-4 fold bigger than those derived from NRAD cells. In tumors derived from RAD cells, the mitotic index was significantly higher than in those derived from NRAD cells, while Ki-67 and survivin expression were more pronounced in RAD tumors. This study suggests that SCC RAD stem cells play a critical role in the formation and development of epithelial tumors.
Figures
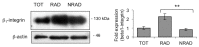





References
-
- Lohmann C.M., Solomon A.R. Clinicopathologic variants of cutaneous squamous cell carcinoma. Adv. Anat. Pathol. 2001;8:27–36. - PubMed
-
- Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplyfying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

