Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity
- PMID: 24077167
- PMCID: PMC3899790
- DOI: 10.1161/CIRCULATIONAHA.113.002887
Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity
Abstract
Background: Smooth muscle cells (SMCs) are remarkably plastic. Their reversible differentiation is required for growth and wound healing but also contributes to pathologies such as atherosclerosis and restenosis. Although key regulators of the SMC phenotype, including myocardin (MYOCD) and KLF4, have been identified, a unifying epigenetic mechanism that confers reversible SMC differentiation has not been reported.
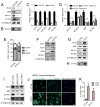
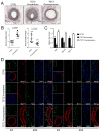
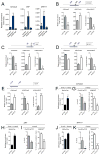
Methods and results: Using human SMCs, human arterial tissue, and mouse models, we report that SMC plasticity is governed by the DNA-modifying enzyme ten-eleven translocation-2 (TET2). TET2 and its product, 5-hydroxymethylcytosine (5-hmC), are enriched in contractile SMCs but reduced in dedifferentiated SMCs. TET2 knockdown inhibits expression of key procontractile genes, including MYOCD and SRF, with concomitant transcriptional upregulation of KLF4. TET2 knockdown prevents rapamycin-induced SMC differentiation, whereas TET2 overexpression is sufficient to induce a contractile phenotype. TET2 overexpression also induces SMC gene expression in fibroblasts. Chromatin immunoprecipitation demonstrates that TET2 coordinately regulates phenotypic modulation through opposing effects on chromatin accessibility at the promoters of procontractile versus dedifferentiation-associated genes. Notably, we find that TET2 binds and 5-hmC is enriched in CArG-rich regions of active SMC contractile promoters (MYOCD, SRF, and MYH11). Loss of TET2 and 5-hmC positively correlates with the degree of injury in murine models of vascular injury and human atherosclerotic disease. Importantly, localized TET2 knockdown exacerbates injury response, and local TET2 overexpression restores the 5-hmC epigenetic landscape and contractile gene expression and greatly attenuates intimal hyperplasia in vivo.
Conclusions: We identify TET2 as a novel and necessary master epigenetic regulator of SMC differentiation.
Keywords: cell differentiation; epigenomics; gene expression regulation; hyperplasia; muscle, smooth.
Conflict of interest statement
Figures




Comment in
-
UnTEThering (smooth muscle) cell plasticity.Circulation. 2013 Oct 29;128(18):2002-4. doi: 10.1161/CIRCULATIONAHA.113.006092. Epub 2013 Sep 27. Circulation. 2013. PMID: 24077168 Free PMC article. No abstract available.
-
Atherosclerosis: cell biology and lipoproteins - epigenetics and oxidation in atherosclerosis.Curr Opin Lipidol. 2014 Jun;25(3):235-6. doi: 10.1097/MOL.0000000000000075. Curr Opin Lipidol. 2014. PMID: 24806894 No abstract available.
-
Letter by Li and Yu regarding article, "ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity".Circulation. 2014 Aug 19;130(8):e71. doi: 10.1161/CIRCULATIONAHA.113.007078. Circulation. 2014. PMID: 25135132 No abstract available.
-
Response to letter regarding article, "ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity".Circulation. 2014 Aug 19;130(8):e72. doi: 10.1161/CIRCULATIONAHA.114.010272. Circulation. 2014. PMID: 25135133 No abstract available.
References
-
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

