Divergent evolution of protein conformational dynamics in dihydrofolate reductase
- PMID: 24077226
- PMCID: PMC3823643
- DOI: 10.1038/nsmb.2676
Divergent evolution of protein conformational dynamics in dihydrofolate reductase
Abstract
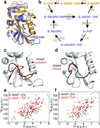
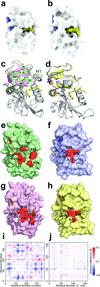
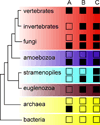
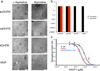
Molecular evolution is driven by mutations, which may affect the fitness of an organism and are then subject to natural selection or genetic drift. Analysis of primary protein sequences and tertiary structures has yielded valuable insights into the evolution of protein function, but little is known about the evolution of functional mechanisms, protein dynamics and conformational plasticity essential for activity. We characterized the atomic-level motions across divergent members of the dihydrofolate reductase (DHFR) family. Despite structural similarity, Escherichia coli and human DHFRs use different dynamic mechanisms to perform the same function, and human DHFR cannot complement DHFR-deficient E. coli cells. Identification of the primary-sequence determinants of flexibility in DHFRs from several species allowed us to propose a likely scenario for the evolution of functionally important DHFR dynamics following a pattern of divergent evolution that is tuned by cellular environment.
Figures


References
-
- Kohen A, Klinman JP. Protein flexibility correlates with degree of hydrogen tunneling in thermophilic and mesophilic alcohol dehydrogenases. J. Am Chem. Soc. 2000;122:10738–10739.
-
- Wolf-Watz M, et al. Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat. Struct. Mol. Biol. 2004;11:945–949. - PubMed
Methods-Only References
-
- Loria JP, Rance M, Palmer AG., III A relaxation-compensated Carr-Purcell-Meiboom-Gill sequence for characterizing chemical exchange by NMR spectroscopy. J. Am Chem. Soc. 1999;121:2331–2332.
-
- Massi F, Johnson E, Wang C, Rance M, Palmer AG., III NMR R1ρ rotating-frame relaxation with weak radio frequency fields. J. Am Chem. Soc. 2004;126:2247–2256. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

