Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy
- PMID: 24077405
- PMCID: PMC6322197
- DOI: 10.1177/107327481302000406
Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy
Abstract
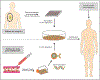
Background: Current FDA-approved therapeutic options for patients with metastatic melanoma include dacarbazine, interleukin 2, ipilimumab, vemurafenib, dabrafenib, and trametinib, but long-term tumor regression using available agents remains out of reach for most patients. Adoptive cell transfer (ACT) with autologous tumor-infiltrating lymphocytes (TILs) has shown encouraging results in clinical trials, with evidence of durable ongoing complete responses in patients with advanced melanoma. Emerging techniques to engineer T-cell receptors (TCRs) or chimeric antigen receptors (CARs) using lymphocytes from peripheral blood may offer new tactics in ACT.
Methods: We reviewed the literature to provide a synopsis on the development and clinical trial results of ACT, as well as the future outlook for using ACT in patients with metastatic melanoma.
Results: ACT with TILs as part of a lymphodepleting regimen has been shown in clinical trials to cause objective clinical responses in approximately 40% to 72% of patients with metastatic melanoma, with up to 40% of those patients experiencing complete responses lasting up to 7 years ongoing. Pilot trials using TCR-engineered cells against melanoma-associated antigens MART-1 and gp100 and the cancer-testis antigen NY-ESO-1 have shown clinical responses in patients with melanoma. CAR cells directed against melanoma have been tested only in preclinical models; however, CAR cells targeting other histologies such as lymphoma have elicited antitumor responses in patients.
Conclusions: An example of state-of-the-art personalized medicine, ACT is a potentially curative therapy for patients with metastatic melanoma. Ongoing trials aiming to simplify the regimens may allow a broader range of patients to be treated and enable ACT to be offered by academic cancer centers.
Figures
References
-
- Sondak VK, Sabel MS, Mulé JJ. Allogeneic and autologous melanoma vaccines: where have we been and where are we going? Clin Cancer Res. 2006;12(7 pt 2):2337s–2341s. - PubMed
-
- Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175(9):6169–6176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

