Reduction in Golgi apparatus dimension in the absence of a residential protein, N-acetylglucosaminyltransferase V
- PMID: 24078077
- PMCID: PMC4085668
- DOI: 10.1007/s00418-013-1146-1
Reduction in Golgi apparatus dimension in the absence of a residential protein, N-acetylglucosaminyltransferase V
Abstract
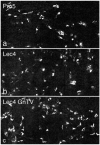
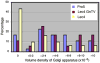
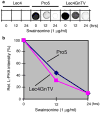
Various proteins are involved in the generation and maintenance of the membrane complex known as the Golgi apparatus. We have used mutant Chinese hamster ovary (CHO) cell lines Lec4 and Lec4A lacking N-acetylglucosaminyltransferase V (GlcNAcT-V, MGAT5) activity and protein in the Golgi apparatus to study the effects of the absence of a single glycosyltransferase on the Golgi apparatus dimension. Quantification of immunofluorescence in serial confocal sections for Golgi α-mannosidase II and electron microscopic morphometry revealed a reduction in Golgi volume density up to 49 % in CHO Lec4 and CHO Lec4A cells compared to parental CHO cells. This reduction in Golgi volume density could be reversed by stable transfection of Lec4 cells with a cDNA encoding Mgat5. Inhibition of the synthesis of β1,6-branched N-glycans by swainsonine had no effect on Golgi volume density. In addition, no effect on Golgi volume density was observed in CHO Lec1 cells that contain enzymatically active GlcNAcT-V, but cannot synthesize β1,6-branched glycans due to an inactive GlcNAcT-I in their Golgi apparatus. These results indicate that it may be the absence of the GlcNAcT-V protein that is the determining factor in reducing Golgi volume density. No dimensional differences existed in cross-sectioned cisternal stacks between Lec4 and control CHO cells, but significantly reduced Golgi stack hits were observed in cross-sectioned Lec4 cells. Therefore, the Golgi apparatus dimensional change in Lec4 and Lec4A cells may be due to a compaction of the organelle.
Figures


References
-
- Acharya U, Malhotra V. Reconstitution of Golgi stacks from vesiculated Golgi membranes in permeabilized cells. Semin Cell Dev Biol. 1996;7:511–516. - PubMed
-
- Allan V. Role of motor proteins in organizing the endoplasmic reticulum and Golgi apparatus. Semin Cell Dev Biol. 1996;7:335–342.
-
- Barr FA, Warren G. Disassembly and reassembly of the Golgi apparatus. Semin Cell Dev Biol. 1996;7:505–510.
-
- Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. - PubMed
-
- Boal F, Guetzoyan L, Sessions RB, Zeghouf M, Spooner RA, Lord JM, Cherfils J, Clarkson GJ, Roberts LM, Stephens DJ. LG186: an inhibitor of GBF1 function that causes Golgi disassembly in human and canine cells. Traffic. 2010;11:1537–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

