Rapid construction of insulated genetic circuits via synthetic sequence-guided isothermal assembly
- PMID: 24078086
- PMCID: PMC3874176
- DOI: 10.1093/nar/gkt860
Rapid construction of insulated genetic circuits via synthetic sequence-guided isothermal assembly
Abstract
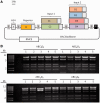
In vitro recombination methods have enabled one-step construction of large DNA sequences from multiple parts. Although synthetic biological circuits can in principle be assembled in the same fashion, they typically contain repeated sequence elements such as standard promoters and terminators that interfere with homologous recombination. Here we use a computational approach to design synthetic, biologically inactive unique nucleotide sequences (UNSes) that facilitate accurate ordered assembly. Importantly, our designed UNSes make it possible to assemble parts with repeated terminator and insulator sequences, and thereby create insulated functional genetic circuits in bacteria and mammalian cells. Using UNS-guided assembly to construct repeating promoter-gene-terminator parts, we systematically varied gene expression to optimize production of a deoxychromoviridans biosynthetic pathway in Escherichia coli. We then used this system to construct complex eukaryotic AND-logic gates for genomic integration into embryonic stem cells. Construction was performed by using a standardized series of UNS-bearing BioBrick-compatible vectors, which enable modular assembly and facilitate reuse of individual parts. UNS-guided isothermal assembly is broadly applicable to the construction and optimization of genetic circuits and particularly those requiring tight insulation, such as complex biosynthetic pathways, sensors, counters and logic gates.
Figures



References
-
- Keasling JD. Synthetic biology for synthetic chemistry. ACS Chem. Biol. 2008;3:64–76. - PubMed
-
- Prather KLJ, Martin CH. De novo biosynthetic pathways: rational design of microbial chemical factories. Curr. Opin. Biotechnol. 2008;19:468–474. - PubMed
-
- Savage DF, Way J, Silver PA. Defossiling fuel: how synthetic biology can transform biofuel production. ACS Chem. Biol. 2008;3:13–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

