Dynactin Deficiency in the CNS of Humans with Sporadic ALS and Mice with Genetically Determined Motor Neuron Degeneration
- PMID: 24078265
- PMCID: PMC3898179
- DOI: 10.1007/s11064-013-1160-7
Dynactin Deficiency in the CNS of Humans with Sporadic ALS and Mice with Genetically Determined Motor Neuron Degeneration
Abstract
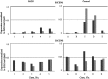
Dynactin is a complex motor protein involved in the retrograde axonal transport disturbances of which may lead to amyotrophic lateral sclerosis (ALS). Mice with hSOD1G93A mutation develop ALS-like symptoms and are used as a model for the disease studies. Similar symptoms demonstrate Cra1 mice, with Dync1h1 mutation. Dynactin heavy (DCTN1) and light (DCTN3) subunits were studied in the CNS of humans with sporadic ALS (SALS), mice with hSOD1G93A (SOD1/+), Dync1h1 (Cra1/+), and double (Cra1/SOD1) mutation at presymptomatic and symptomatic stages. In SALS subjects, in contrast to control cases, expression of DCTN1-mRNA but not DCTN3-mRNA in the motor cortex was higher than in the sensory cortex. However, the mean levels of DCTN1-mRNA and protein were lower in both SALS cortexes and in the spinal cord than in control structures. DCTN3 was unchanged in brain cortexes but decreased in the spinal cord on both mRNA and protein levels. In all SALS tissues immunohistochemical analyses revealed degeneration and loss of neuronal cells, and poor expression of dynactin subunits. In SOD1/+ mice both subunits expression was significantly lower in the frontal cortex, spinal cord and hippocampus than in wild-type controls, especially at presymptomatic stage. Fewer changes occurred in Cra1/SOD1 and Cra1/+ mice.It can be concluded that in sporadic and SOD1-related ALS the impairment of axonal retrograde transport may be due to dynactin subunits deficiency and subsequent disturbances of the whole dynein/dynactin complex structure and function. The Dync1h1 mutation itself has slight negative effect on dynactin expression and it alleviates the changes caused by SOD1G93A mutation.
Figures




Similar articles
-
Differences in glutathione S-transferase pi expression in transgenic mice with symptoms of neurodegeneration.Acta Biochim Pol. 2011;58(4):621-6. Epub 2011 Nov 30. Acta Biochim Pol. 2011. PMID: 22132373
-
Kinesin expression in the central nervous system of humans and transgenic hSOD1G93A mice with amyotrophic lateral sclerosis.Neurodegener Dis. 2013;12(2):71-80. doi: 10.1159/000339529. Epub 2012 Sep 21. Neurodegener Dis. 2013. PMID: 23006449
-
Changes in kinesin expression in the CNS of mice with dynein heavy chain 1 mutation.Acta Biochim Pol. 2013;60(1):51-5. Epub 2013 Mar 5. Acta Biochim Pol. 2013. PMID: 23460941
-
Neuropathology and omics in motor neuron diseases.Neuropathology. 2012 Aug;32(4):458-62. doi: 10.1111/j.1440-1789.2011.01281.x. Epub 2011 Dec 22. Neuropathology. 2012. PMID: 22187969 Review.
-
[Role of axonal transport in ALS].Rinsho Shinkeigaku. 2011 Nov;51(11):1189-91. doi: 10.5692/clinicalneurol.51.1189. Rinsho Shinkeigaku. 2011. PMID: 22277530 Review. Japanese.
Cited by
-
Temperature- and chemical-induced neurotoxicity in zebrafish.Front Physiol. 2023 Oct 3;14:1276941. doi: 10.3389/fphys.2023.1276941. eCollection 2023. Front Physiol. 2023. PMID: 37854466 Free PMC article. Review.
-
The role of autophagy in the pathogenesis and treatment of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD).Autophagy Rep. 2025 Mar 20;4(1):2474796. doi: 10.1080/27694127.2025.2474796. eCollection 2025. Autophagy Rep. 2025. PMID: 40395983 Free PMC article. Review.
-
Dynactin1 depletion leads to neuromuscular synapse instability and functional abnormalities.Mol Neurodegener. 2019 Jul 10;14(1):27. doi: 10.1186/s13024-019-0327-3. Mol Neurodegener. 2019. PMID: 31291987 Free PMC article.
-
Alternative Splicing of ALS Genes: Misregulation and Potential Therapies.Cell Mol Neurobiol. 2020 Jan;40(1):1-14. doi: 10.1007/s10571-019-00717-0. Epub 2019 Aug 5. Cell Mol Neurobiol. 2020. PMID: 31385134 Free PMC article. Review.
-
Genetic and Transcriptomic Biomarkers in Neurodegenerative Diseases: Current Situation and the Road Ahead.Cells. 2021 Apr 27;10(5):1030. doi: 10.3390/cells10051030. Cells. 2021. PMID: 33925602 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

