Stress signaling pathways for the pathogenicity of Cryptococcus
- PMID: 24078305
- PMCID: PMC3889573
- DOI: 10.1128/EC.00218-13
Stress signaling pathways for the pathogenicity of Cryptococcus
Abstract
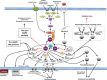
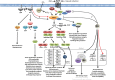
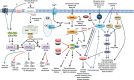
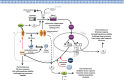
Sensing, responding, and adapting to the surrounding environment are crucial for all living organisms to survive, proliferate, and differentiate in their biological niches. This ability is also essential for Cryptococcus neoformans and its sibling species Cryptococcus gattii, as these pathogens have saprobic and parasitic life cycles in natural and animal host environments. The ability of Cryptococcus to cause fatal meningoencephalitis is highly related to its capability to remodel and optimize its metabolic and physiological status according to external cues. These cues act through multiple stress signaling pathways through a panoply of signaling components, including receptors/sensors, small GTPases, secondary messengers, kinases, transcription factors, and other miscellaneous adaptors or regulators. In this minireview, we summarize and highlight the importance of several stress signaling pathways that influence the pathogenicity of Cryptococcus and discuss future challenges in these areas.
Figures


References
-
- Price MS, Perfect JR. 2011. Host defenses against cryptococcosis. Immunol. Invest. 40:786–808 - PubMed
-
- Perfect JR. 2012. The impact of the host on fungal infections. Am. J. Med. 125:S39–S51 - PubMed
-
- Kwon-Chung KJ, Sorrell TC, Dromer F, Fung E, Levitz SM. 2000. Cryptococcosis: clinical and biological aspects. Med. Mycol. 38(Suppl 1):205–213 - PubMed
-
- Lin X, Heitman J. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60:69–105 - PubMed
-
- Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, Heitman J. 2005. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3:753–764 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

