Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage
- PMID: 24078628
- PMCID: PMC3829152
- DOI: 10.1074/jbc.M113.517540
Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage
Abstract
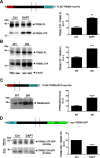
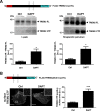
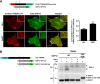
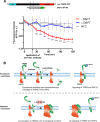
Triggering receptor expressed on myeloid cells-2 (TREM2) and its signaling adaptor protein TYROBP/DAP12 play important roles in signal transduction in dendritic cells, osteoclasts, tissue macrophages, and microglia. Recently, TREM2 variants have been shown to be linked to late onset Alzheimer disease. Here, we demonstrate that TREM2 undergoes sequential proteolytic processing by ectodomain shedding and intramembrane proteolysis. The C-terminal fragment (CTF) of TREM2 generated by ectodomain shedding is cleaved by γ-secretase. Importantly, pharmacologic and genetic γ-secretase inhibition resulted in accumulation of TREM2 CTF at the plasma membrane that also interacts with the signaling adaptor protein DAP12. Thus, the accumulated TREM2 CTF thereby might limit the interaction of DAP12 with the functional full-length receptor, resulting in decreased DAP12 phosphorylation and impaired metabolism of phosphatidylinositol 4,5-bisphosphate. Together, these data demonstrate γ-secretase-mediated intramembranous proteolysis of TREM2 and functionally link two Alzheimer disease-associated proteins in one signaling pathway.
Keywords: Alzheimer Disease; Intramembrane Proteolysis; Membrane; Presenilin; Protein Phosphorylation; Secretases; Signaling.
Figures

References
-
- Colonna M. (2003) TREMs in the immune system and beyond. Nat. Rev. Immunol. 3, 445–453 - PubMed
-
- Bouchon A., Dietrich J., Colonna M. (2000) Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 - PubMed
-
- Sessa G., Podini P., Mariani M., Meroni A., Spreafico R., Sinigaglia F., Colonna M., Panina P., Meldolesi J. (2004) Distribution and signalling of TREM2/DAP12, the receptor system mutated in the human PLOSL dementia. Eur. J. Neurosci. 20, 2617–2628 - PubMed
-
- Prada I., Ongania G. N., Buonsanti C., Panina-Bordignon P., Meldolesi J. (2006) Triggering receptor expressed in myeloid cells 2 (TREM2) trafficking in microglial cells: continuous shuttling to and from the plasma membrane regulated by cell stimulation. Neuroscience 140, 1139–1148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

