Purinergic signalling: from discovery to current developments
- PMID: 24078669
- PMCID: PMC4208685
- DOI: 10.1113/expphysiol.2013.071951
Purinergic signalling: from discovery to current developments
Abstract
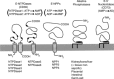
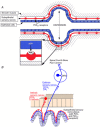
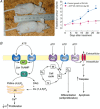
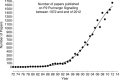
This lecture is about the history of the purinergic signalling concept. It begins with reference to the paper by Paton & Vane published in 1963, which identified non-cholinergic relaxation in response to vagal nerve stimulation in several species, although they suggested that it might be due to sympathetic adrenergic nerves in the vagal nerve trunk. Using the sucrose gap technique for simultaneous mechanical and electrical recordings in smooth muscle (developed while in Feldberg's department in the National Institute for Medical Research) of the guinea-pig taenia coli preparation (learned when working in Edith Bülbring's smooth muscle laboratory in Oxford Pharmacology), we showed that the hyperpolarizations recorded in the presence of antagonists to the classical autonomic neurotransmitters, acetylcholine and noradrenaline, were inhibitory junction potentials in response to non-adrenergic, non-cholinergic neurotransmission, mediated by intrinsic enteric nerves controlled by vagal and sacral parasympathetic nerves. We then showed that ATP satisfied the criteria needed to identify a neurotransmitter released by these nerves. Subsequently, it was shown that ATP is a cotransmitter in all nerves in the peripheral and central nervous systems. The receptors for purines and pyrimidines were cloned and characterized in the early 1990 s, and immunostaining showed that most non-neuronal cells as well as nerve cells expressed these receptors. The physiology and pathophysiology of purinergic signalling is discussed.
Figures











References
-
- Abbracchio MP. Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol Ther. 1994;64:445–475. - PubMed
-
- Abbracchio MP. Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. - PubMed
-
- Agboh KC, Webb TE, Evans RJ. Ennion SJ. Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem. 2004;279:41650–41657. - PubMed
-
- Abbracchio MP, Burnstock G, Verkhratsky A. Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. - PubMed
-
- Bailey MA, Shirley DG, King BF, Burnstock G, Unwin RJ. Extracellular nucleotides and renal function. In: Alpern RJ, Herbert SCG, editors. The Kidney: Physiology and Pathophysiology. San Diego: Elsevier; 2007. pp. 425–443.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

