Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages
- PMID: 24078688
- PMCID: PMC3808250
- DOI: 10.4049/jimmunol.1300581
Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages
Abstract
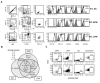
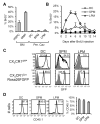
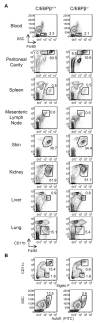
Macrophages and dendritic cells (DC) are distributed throughout the body and play important roles in pathogen detection and tissue homeostasis. In tissues, resident macrophages exhibit distinct phenotypes and activities, yet the transcriptional pathways that specify tissue-specific macrophages are largely unknown. We investigated the functions and origins of two peritoneal macrophage populations in mice: small and large peritoneal macrophages (SPM and LPM, respectively). SPM and LPM differ in their ability to phagocytose apoptotic cells, as well as in the production of cytokines in response to LPS. In steady-state conditions, SPM are sustained by circulating precursors, whereas LPM are maintained independently of hematopoiesis; however, both populations are replenished by bone marrow precursors following radiation injury. Transcription factor analysis revealed that SPM and LPM express abundant CCAAT/enhancer binding protein (C/EBP)-β. Cebpb(-/-) mice exhibit elevated numbers of SPM-like cells but lack functional LPM. Alveolar macrophages are also missing in Cebpb(-/-) mice, although macrophage populations in the spleen, kidney, skin, mesenteric lymph nodes, and liver are normal. Adoptive transfer of SPM into Cebpb(-/-) mice results in SPM differentiation into LPM, yet donor SPM do not generate LPM after transfer into C/EBPβ-sufficient mice, suggesting that endogenous LPM inhibit differentiation by SPM. We conclude that C/EBPβ plays an intrinsic, tissue-restricted role in the generation of resident macrophages.
Figures



References
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
-
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

