Reporting of harms data in RCTs: a systematic review of empirical assessments against the CONSORT harms extension
- PMID: 24078752
- PMCID: PMC3787508
- DOI: 10.1136/bmjopen-2013-003436
Reporting of harms data in RCTs: a systematic review of empirical assessments against the CONSORT harms extension
Abstract
Objective: To determine the standard of reporting of harms-related data, in randomised controlled trials (RCTs) according to the Consolidated Standards of Reporting Trials (CONSORT) statement extension for harms.
Design: Systematic review.
Data sources: The Cochrane library, Ovid MEDLINE, Scopus and ISI Web of Knowledge were searched for relevant literature.
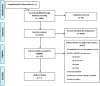
Eligibility criteria for selecting studies: We included publications of studies that used the CONSORT harms extension to assess the reporting of harms in RCTs.
Results: We identified 7 studies which included between 10 and 205 RCTs. The clinical areas of the 7 studies were: hypertension (1), urology (1), epilepsy (1), complimentary medicine (2) and two not restricted to a clinical topic. Quality of the 7 studies was assessed by a risk of bias tool and was found to be variable. Adherence to the CONSORT harms criteria reported in the 7 studies was inadequate and variable across the items in the checklist. Adverse events are poorly defined, with 6 studies failing to exceed 50% adherence to the items in the checklist.
Conclusions: Readers of RCT publications need to be able to balance the trade-offs between benefits and harms of interventions. This systematic review suggests that this is compromised due to poor reporting of harms which is evident across a range of clinical areas. Improvements in quality could be achieved by wider adoption of the CONSORT harms criteria by journals reporting RCTs.
Keywords: Biostatistics; Clinical Trial Methodology; General Medicine (see Internal Medicine).
Figures
References
-
- Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001;285:437–43 - PubMed
-
- Moher D, Jones A, Lepage L, et al. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA 2001;285:1992–5 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases