A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors
- PMID: 24078773
- PMCID: PMC3864571
- DOI: 10.1158/2159-8290.CD-13-0183
A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors
Abstract
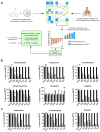
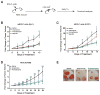
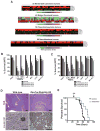
Small cell lung cancer (SCLC) is an aggressive neuroendocrine subtype of lung cancer with high mortality. We used a systematic drug repositioning bioinformatics approach querying a large compendium of gene expression profiles to identify candidate U.S. Food and Drug Administration (FDA)-approved drugs to treat SCLC. We found that tricyclic antidepressants and related molecules potently induce apoptosis in both chemonaïve and chemoresistant SCLC cells in culture, in mouse and human SCLC tumors transplanted into immunocompromised mice, and in endogenous tumors from a mouse model for human SCLC. The candidate drugs activate stress pathways and induce cell death in SCLC cells, at least in part by disrupting autocrine survival signals involving neurotransmitters and their G protein-coupled receptors. The candidate drugs inhibit the growth of other neuroendocrine tumors, including pancreatic neuroendocrine tumors and Merkel cell carcinoma. These experiments identify novel targeted strategies that can be rapidly evaluated in patients with neuroendocrine tumors through the repurposing of approved drugs.
Significance: Our work shows the power of bioinformatics-based drug approaches to rapidly repurpose FDA-approved drugs and identifies a novel class of molecules to treat patients with SCLC, a cancer for which no effective novel systemic treatments have been identified in several decades. In addition, our experiments highlight the importance of novel autocrine mechanisms in promoting the growth of neuroendocrine tumor cells.
©2013 AACR.
Conflict of interest statement
Figures



Comment in
-
Teaching an old dog new tricks: drug repositioning in small cell lung cancer.Cancer Discov. 2013 Dec;3(12):1333-5. doi: 10.1158/2159-8290.CD-13-0790. Cancer Discov. 2013. PMID: 24327694 Free PMC article.
References
-
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nature reviews Drug discovery. 2004;3:673–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

