In vivo dynamics of innate immune sentinels in the CNS
- PMID: 24078900
- PMCID: PMC3784260
- DOI: 10.4161/intv.22823
In vivo dynamics of innate immune sentinels in the CNS
Abstract
The innate immune system is comprised of cellular sentinels that often serve as the first responders to injury and invading pathogens. Our basic understanding of innate immunity is derived from research conducted in peripheral lymphoid tissues. However, it is now recognized that most non-lymphoid tissues throughout the body are equipped with specialized innate immune cells that are uniquely adapted to the niches in which they reside. The central nervous system (CNS) is a particularly interesting compartment because it contains a population of post-mitotic cells (neurons) that are intolerant of robust, cytopathic inflammatory responses observed in many peripheral tissues. Thus, evolutionary adaptations have fitted the CNS with a unique array of innate immune sentinels that facilitate the development of local inflammatory responses but attempt to do so in a manner that preserves the integrity of its post-mitotic residents. Interestingly, studies have even suggested that CNS resident innate immune cells contribute to the homeostasis of this compartment and promote neural activity. In this review we discuss recent advances in our understanding of CNS innate immune sentinels and how novel imaging approaches such as intravital two-photon laser scanning microscopy (TPLSM) have shed light on these cells during states of health and disease.
Keywords: CNS; dendritic cells; innate immunity; macrophages; microglia.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
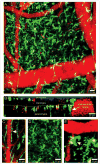
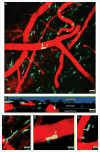

References
-
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–62. http://dx.doi.org/10.1038/nature09615. - DOI - PubMed
-
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–35. http://dx.doi.org/10.1038/nn.2923. - DOI - PubMed
-
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. http://dx.doi.org/10.1038/nri3265. - DOI - PubMed
-
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. http://dx.doi.org/10.1126/science.1194637. - DOI - PMC - PubMed
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. http://dx.doi.org/10.1126/science.1110647. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
