Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: paracellular, transcellular or both?
- PMID: 24079544
- PMCID: PMC3850506
- DOI: 10.1186/1478-811X-11-72
Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: paracellular, transcellular or both?
Abstract
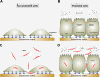
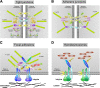
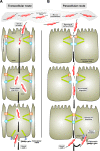
Intact intercellular junctions and cellular matrix contacts are crucial structural components for the formation and maintenance of epithelial barrier functions in humans to control the commensal flora and protect against intruding microbes. Campylobacter jejuni is one of the most important zoonotic pathogens causing food-borne gastroenteritis and potentially more severe diseases such as reactive arthritis or Guillain-Barré syndrome. Crossing the intestinal epithelial barrier and host cell invasion by C. jejuni are considered to represent the primary reasons of gut tissue damage in humans and various animal model systems including monkeys, piglets, rabbits, hamsters and ferrets. C. jejuni is also able to invade underlying tissues such as the lamina propria, can enter the bloodstream, and possibly reach distinct organs such as spleen, liver or mesenteric lymph nodes. However, the molecular mechanisms as well as major bacterial and host cell factors involved in these activities are poorly understood. Various models exist by which the pathogen can trigger its own transmigration across polarized intestinal epithelial cells in vitro, the paracellular and/or transcellular mechanism. Recent studies suggest that bacterial factors such as flagellum, serine protease HtrA and lipooligosaccharide LOS may play an active role in bacterial transmigration. Here we review our knowledge on transmigration of C. jejuni as well as some other Campylobacter species, and discuss the pros and cons for the route(s) taken to travel across polarized epithelial cell monolayers. These studies provide fresh insights into the infection strategies employed by this important pathogen.
Figures
References
-
- Friedman CR, Neimann J, Wegener HC, Tauxe RV. In: Campylobacter. Nachamkin I, Blaser MJ, editor. Washington, DC: ASM Press; 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations; pp. 121–138.
-
- Oyarzabal OA, Backert S. Microbial Food Safety. New York: Springer; 2011.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

