Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory
- PMID: 24079852
- PMCID: PMC4153951
- DOI: 10.1111/bjd.12636
Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory
Abstract
Background: Psoriasis symptoms have a significant negative impact on health-related quality of life, impairing physical functioning and well-being.
Objective: To evaluate the impact of brodalumab, a human anti-interleukin-17R monoclonal antibody, on psoriasis symptom severity as measured by a novel patient-reported outcome measure, the Psoriasis Symptom Inventory, and dermatology-specific health-related quality of life as measured by the Dermatology Life Quality Index (DLQI).
Methods: This was a secondary analysis of a phase II, randomized, double-blind, placebo-controlled clinical study of patients with moderate-to-severe psoriasis (n = 198) treated with brodalumab or placebo. This analysis assessed Psoriasis Symptom Inventory scores and DLQI scores over time. Analyses were conducted on all patients who were randomized and received one or more injections of the study drug according to intention to treat using last observation carried forward to impute missing data.
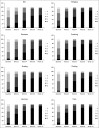
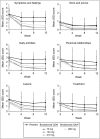
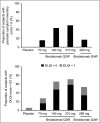
Results: At week 12, subjects in the brodalumab groups had significant improvements in mean Psoriasis Symptom Inventory total scores [8.5 (70 mg), 15.8 (140 mg), 16.2 (210 mg) and 12.7 (280 mg)] compared with placebo (4.8). Mean improvements in DLQI were clinically meaningful (≥ 5.7) in the brodalumab groups (6.2, 9.1, 9.6 and 7.1, respectively) and significantly greater than placebo (3.1). Improvements in Psoriasis Symptom Inventory were observed as early as week 2 and in DLQI by week 4. All eight Psoriasis Symptom Inventory item scores improved significantly among the brodalumab groups by week 12.
Conclusions: Results were from a single randomized clinical trial and may not generalize to broader patient populations. However, treatment with brodalumab provided significant improvement in psoriasis symptoms in patients with moderate-to-severe psoriasis.
Trial registration: ClinicalTrials.gov NCT00975637.
© 2013 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures


References
-
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346–9. - PubMed
-
- Yosipovitch G, Goon A, Wee J, et al. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–73. - PubMed
-
- Sampogna F, Sera F, Abeni D. Measures of clinical severity, quality of life, and psychological distress in patients with psoriasis: a cluster analysis. J Invest Dermatol. 2004;122:602–7. - PubMed
-
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

