Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/β-catenin signaling
- PMID: 24080158
- PMCID: PMC3953133
- DOI: 10.1016/j.cellsig.2013.09.016
Secreted Frizzled-related protein potentiation versus inhibition of Wnt3a/β-catenin signaling
Abstract
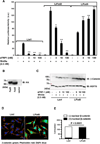
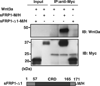
Wnt signaling regulates a variety of cellular processes during embryonic development and in the adult. Many of these activities are mediated by the Frizzled family of seven-pass transmembrane receptors, which bind Wnts via a conserved cysteine-rich domain (CRD). Secreted Frizzled-related proteins (sFRPs) contain an amino-terminal, Frizzled-like CRD and a carboxyl-terminal, heparin-binding netrin-like domain. Previous studies identified sFRPs as soluble Wnt antagonists that bind directly to Wnts and prevent their interaction with Frizzleds. However, subsequent observations suggested that sFRPs and Frizzleds form homodimers and heterodimers via their respective CRDs, and that sFRPs can stimulate signal transduction. Here, we present evidence that sFRP1 either inhibits or enhances signaling in the Wnt3a/β-catenin pathway, depending on its concentration and the cellular context. Nanomolar concentrations of sFRP1 increased Wnt3a signaling, while higher concentrations blocked it in HEK293 cells expressing a SuperTopFlash reporter. sFRP1 primarily augmented Wnt3a/β-catenin signaling in C57MG cells, but it behaved as an antagonist in L929 fibroblasts. sFRP1 enhanced reporter activity in L cells that were engineered to stably express Frizzled 5, though not Frizzled 2. This implied that the Frizzled expression pattern could determine the response to sFRP1. Similar results were obtained with sFRP2 in HEK293, C57MG and L cell reporter assays. CRDsFRP1 mimicked the potentiating effect of sFRP1 in multiple settings, contradicting initial expectations that this domain would inhibit Wnt signaling. Moreover, CRDsFRP1 showed little avidity for Wnt3a compared to sFRP1, implying that the mechanism for potentiation by CRDsFRP1 probably does not require an interaction with Wnt protein. Together, these findings demonstrate that sFRPs can either promote or suppress Wnt/β-catenin signaling, depending on cellular context, concentration and most likely the expression pattern of Fzd receptors.
Keywords: BSA; CRD; Cysteine-rich domain; Frizzled; Fzd; PBS; Wnt; bovine serum albumin; cysteine-rich domain; phosphate-buffered saline; sFRP; secreted Frizzled-related protein; β-Catenin.
© 2013.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

