Genetic modifiers of menopausal hormone replacement therapy and breast cancer risk: a genome-wide interaction study
- PMID: 24080446
- PMCID: PMC3863710
- DOI: 10.1530/ERC-13-0349
Genetic modifiers of menopausal hormone replacement therapy and breast cancer risk: a genome-wide interaction study
Abstract
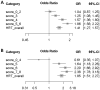
Women using menopausal hormone therapy (MHT) are at increased risk of developing breast cancer (BC). To detect genetic modifiers of the association between current use of MHT and BC risk, we conducted a meta-analysis of four genome-wide case-only studies followed by replication in 11 case-control studies. We used a case-only design to assess interactions between single-nucleotide polymorphisms (SNPs) and current MHT use on risk of overall and lobular BC. The discovery stage included 2920 cases (541 lobular) from four genome-wide association studies. The top 1391 SNPs showing P values for interaction (Pint) <3.0 × 10(-3) were selected for replication using pooled case-control data from 11 studies of the Breast Cancer Association Consortium, including 7689 cases (676 lobular) and 9266 controls. Fixed-effects meta-analysis was used to derive combined Pint. No SNP reached genome-wide significance in either the discovery or combined stage. We observed effect modification of current MHT use on overall BC risk by two SNPs on chr13 near POMP (combined Pint≤8.9 × 10(-6)), two SNPs in SLC25A21 (combined Pint≤4.8 × 10(-5)), and three SNPs in PLCG2 (combined Pint≤4.5 × 10(-5)). The association between lobular BC risk was potentially modified by one SNP in TMEFF2 (combined Pint≤2.7 × 10(-5)), one SNP in CD80 (combined Pint≤8.2 × 10(-6)), three SNPs on chr17 near TMEM132E (combined Pint≤2.2×10(-6)), and two SNPs on chr18 near SLC25A52 (combined Pint≤4.6 × 10(-5)). In conclusion, polymorphisms in genes related to solute transportation in mitochondria, transmembrane signaling, and immune cell activation are potentially modifying BC risk associated with current use of MHT. These findings warrant replication in independent studies.
Keywords: breast cancer; genetic variation; genome-wide; menopausal hormone therapy.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
-
- Andersen SW, Trentham-Dietz A, Figueroa JD, Titus LJ, Cai Q, Long J, Hampton JM, Egan KM, Newcomb PA. Breast cancer susceptibility associated with rs1219648 (fibroblast growth factor receptor 2) and postmenopausal hormone therapy use in a population-based United States study. Menopause 2012 - PMC - PubMed
-
- Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, Clavel-Chapelon F, Fabre A, Hemon B, Rinaldi S, Chajes V, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128:144–156. - PubMed
-
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. - PubMed
-
- Bhatia S, Edidin M, Almo SC, Nathenson SG. B7-1 and B7-2: similar costimulatory ligands with different biochemical, oligomeric and signaling properties. Immunol Lett. 2006;104:70–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA050385/CA/NCI NIH HHS/United States
- CA128978/CA/NCI NIH HHS/United States
- C1287/A10710/CRUK_/Cancer Research UK/United Kingdom
- R01 CA067262/CA/NCI NIH HHS/United States
- 16565/CRUK_/Cancer Research UK/United Kingdom
- BREAST CANCER NOW RESEARCH CENTRE/BBC_/Breast Cancer Now/United Kingdom
- R01 CA128978/CA/NCI NIH HHS/United States
- U19 CA148537/CA/NCI NIH HHS/United States
- P50 CA116201/CA/NCI NIH HHS/United States
- C5047/A15007/CRUK_/Cancer Research UK/United Kingdom
- U01 CA049449/CA/NCI NIH HHS/United States
- 10118/CRUK_/Cancer Research UK/United Kingdom
- C1287/A10118/CRUK_/Cancer Research UK/United Kingdom
- CA65725/CA/NCI NIH HHS/United States
- 5UO1CA098233/CA/NCI NIH HHS/United States
- CA116201/CA/NCI NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- UM1 CA164920/CA/NCI NIH HHS/United States
- 11022/CRUK_/Cancer Research UK/United Kingdom
- CAPMC/ CIHR/Canada
- CA122340/CA/NCI NIH HHS/United States
- C12292/A11174/CRUK_/Cancer Research UK/United Kingdom
- CA87969/CA/NCI NIH HHS/United States
- 1U19 CA148065/CA/NCI NIH HHS/United States
- C5047/A10692/CRUK_/Cancer Research UK/United Kingdom
- CA49449/CA/NCI NIH HHS/United States
- 1U19 CA148537/CA/NCI NIH HHS/United States
- U01 CA067262/CA/NCI NIH HHS/United States
- R01 CA065725/CA/NCI NIH HHS/United States
- CA67262/CA/NCI NIH HHS/United States
- C5047/A8384/CRUK_/Cancer Research UK/United Kingdom
- U01 CA098233/CA/NCI NIH HHS/United States
- 16563/CRUK_/Cancer Research UK/United Kingdom
- 1U19 CA148112/CA/NCI NIH HHS/United States
- U19 CA148112/CA/NCI NIH HHS/United States
- U19 CA148065/CA/NCI NIH HHS/United States
- R01 CA122340/CA/NCI NIH HHS/United States
- R01 CA049449/CA/NCI NIH HHS/United States
- CA50385/CA/NCI NIH HHS/United States
- C1281/A12014/CRUK_/Cancer Research UK/United Kingdom
- 16561/CRUK_/Cancer Research UK/United Kingdom
- 10124/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical