Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application
- PMID: 24080962
- PMCID: PMC3941007
- DOI: 10.1088/0031-9155/58/20/7391
Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application
Abstract
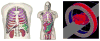
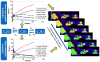
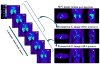
Static whole-body PET/CT, employing the standardized uptake value (SUV), is considered the standard clinical approach to diagnosis and treatment response monitoring for a wide range of oncologic malignancies. Alternative PET protocols involving dynamic acquisition of temporal images have been implemented in the research setting, allowing quantification of tracer dynamics, an important capability for tumor characterization and treatment response monitoring. Nonetheless, dynamic protocols have been confined to single-bed-coverage limiting the axial field-of-view to ~15-20 cm, and have not been translated to the routine clinical context of whole-body PET imaging for the inspection of disseminated disease. Here, we pursue a transition to dynamic whole-body PET parametric imaging, by presenting, within a unified framework, clinically feasible multi-bed dynamic PET acquisition protocols and parametric imaging methods. We investigate solutions to address the challenges of: (i) long acquisitions, (ii) small number of dynamic frames per bed, and (iii) non-invasive quantification of kinetics in the plasma. In the present study, a novel dynamic (4D) whole-body PET acquisition protocol of ~45 min total length is presented, composed of (i) an initial 6 min dynamic PET scan (24 frames) over the heart, followed by (ii) a sequence of multi-pass multi-bed PET scans (six passes × seven bed positions, each scanned for 45 s). Standard Patlak linear graphical analysis modeling was employed, coupled with image-derived plasma input function measurements. Ordinary least squares Patlak estimation was used as the baseline regression method to quantify the physiological parameters of tracer uptake rate Ki and total blood distribution volume V on an individual voxel basis. Extensive Monte Carlo simulation studies, using a wide set of published kinetic FDG parameters and GATE and XCAT platforms, were conducted to optimize the acquisition protocol from a range of ten different clinically acceptable sampling schedules examined. The framework was also applied to six FDG PET patient studies, demonstrating clinical feasibility. Both simulated and clinical results indicated enhanced contrast-to-noise ratios (CNRs) for Ki images in tumor regions with notable background FDG concentration, such as the liver, where SUV performed relatively poorly. Overall, the proposed framework enables enhanced quantification of physiological parameters across the whole body. In addition, the total acquisition length can be reduced from 45 to ~35 min and still achieve improved or equivalent CNR compared to SUV, provided the true Ki contrast is sufficiently high. In the follow-up companion paper, a set of advanced linear regression schemes is presented to particularly address the presence of noise, and attempt to achieve a better trade-off between the mean-squared error and the CNR metrics, resulting in enhanced task-based imaging.
Figures







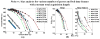


References
-
- Adams M, Turkington T, Wilson J, Wong T. A systematic review of the factors affecting accuracy of SUV measurements. American Journal of Roentgenology. 2010;195(2):310–320. - PubMed
-
- Allen D. Mean Square Error of Prediction as a Criterion for Selecting Variables. Technometrics. 1971;13(3):469–475.
-
- Bergstorm M, Eriksson L, Bohn C, Blomqvist G, Litton J. Correction for scattered radiation in a ring detector positron camera by integral transformation of the projections. J Comput Assist Tomogr. 1983;7:42–50. - PubMed
-
- Burger I, Burger C, Berthold T, Buck A. Simplified quantification of FDG metabolism in tumors using the autoradiographic method is less dependent on the acquisition time than SUV. Nuclear Medicine and Biology. 2011;38:835–841. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
