The yeast AMPK homolog SNF1 regulates acetyl coenzyme A homeostasis and histone acetylation
- PMID: 24081331
- PMCID: PMC3838016
- DOI: 10.1128/MCB.00198-13
The yeast AMPK homolog SNF1 regulates acetyl coenzyme A homeostasis and histone acetylation
Abstract
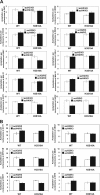
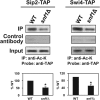
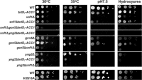
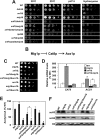
Acetyl coenzyme A (acetyl-CoA) is a key metabolite at the crossroads of metabolism, signaling, chromatin structure, and transcription. Concentration of acetyl-CoA affects histone acetylation and links intermediary metabolism and transcriptional regulation. Here we show that SNF1, the budding yeast ortholog of the mammalian AMP-activated protein kinase (AMPK), plays a role in the regulation of acetyl-CoA homeostasis and global histone acetylation. SNF1 phosphorylates and inhibits acetyl-CoA carboxylase, which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the first and rate-limiting reaction in the de novo synthesis of fatty acids. Inactivation of SNF1 results in a reduced pool of cellular acetyl-CoA, globally decreased histone acetylation, and reduced fitness and stress resistance. The histone acetylation and transcriptional defects can be partially suppressed and the overall fitness improved in snf1Δ mutant cells by increasing the cellular concentration of acetyl-CoA, indicating that the regulation of acetyl-CoA homeostasis represents another mechanism in the SNF1 regulatory repertoire.
Figures





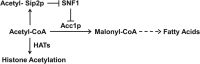
Similar articles
-
Activation of AMP-activated Protein Kinase by Metformin Induces Protein Acetylation in Prostate and Ovarian Cancer Cells.J Biol Chem. 2016 Nov 25;291(48):25154-25166. doi: 10.1074/jbc.M116.742247. Epub 2016 Oct 12. J Biol Chem. 2016. PMID: 27733682 Free PMC article.
-
Yeast phospholipase C is required for normal acetyl-CoA homeostasis and global histone acetylation.J Biol Chem. 2013 Sep 27;288(39):27986-98. doi: 10.1074/jbc.M113.492348. Epub 2013 Aug 2. J Biol Chem. 2013. PMID: 23913687 Free PMC article.
-
Acetyl-CoA carboxylase regulates global histone acetylation.J Biol Chem. 2012 Jul 6;287(28):23865-76. doi: 10.1074/jbc.M112.380519. Epub 2012 May 11. J Biol Chem. 2012. PMID: 22580297 Free PMC article.
-
Reciprocal Regulation of AMPK/SNF1 and Protein Acetylation.Int J Mol Sci. 2018 Oct 25;19(11):3314. doi: 10.3390/ijms19113314. Int J Mol Sci. 2018. PMID: 30366365 Free PMC article. Review.
-
AMPK/Snf1 signaling regulates histone acetylation: Impact on gene expression and epigenetic functions.Cell Signal. 2016 Aug;28(8):887-95. doi: 10.1016/j.cellsig.2016.03.009. Epub 2016 Mar 20. Cell Signal. 2016. PMID: 27010499 Review.
Cited by
-
Inactivation of the transcription factor mig1 (YGL035C) in Saccharomyces cerevisiae improves tolerance towards monocarboxylic weak acids: acetic, formic and levulinic acid.J Ind Microbiol Biotechnol. 2018 Aug;45(8):735-751. doi: 10.1007/s10295-018-2053-1. Epub 2018 Jun 6. J Ind Microbiol Biotechnol. 2018. PMID: 29876685
-
Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: the emerging picture.Front Plant Sci. 2014 Apr 1;5:119. doi: 10.3389/fpls.2014.00119. eCollection 2014. Front Plant Sci. 2014. PMID: 24744765 Free PMC article. Review.
-
Signal transduction: From the atomic age to the post-genomic era.Cold Spring Harb Perspect Biol. 2014 Oct 30;6(12):a022913. doi: 10.1101/cshperspect.a022913. Cold Spring Harb Perspect Biol. 2014. PMID: 25359498 Free PMC article. Review.
-
The effects of TORC signal interference on lipogenesis in the oleaginous yeast Trichosporon oleaginosus.BMC Biotechnol. 2017 Mar 7;17(1):27. doi: 10.1186/s12896-017-0348-3. BMC Biotechnol. 2017. PMID: 28270203 Free PMC article.
-
Perturbed fatty-acid metabolism is linked to localized chromatin hyperacetylation, increased stress-response gene expression and resistance to oxidative stress.PLoS Genet. 2023 Jan 10;19(1):e1010582. doi: 10.1371/journal.pgen.1010582. eCollection 2023 Jan. PLoS Genet. 2023. PMID: 36626368 Free PMC article.
References
-
- Hardie DG, Carling D, Carlson M. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67:821–855 - PubMed
-
- Hardie DG. 2007. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8:774–785 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
