Preoperative angiotensin-converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery
- PMID: 24081864
- PMCID: PMC3811062
- DOI: 10.1093/ndt/gft405
Preoperative angiotensin-converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery
Abstract
Background: Using either an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) the morning of surgery may lead to 'functional' postoperative acute kidney injury (AKI), measured by an abrupt increase in serum creatinine. Whether the same is true for 'structural' AKI, measured with new urinary biomarkers, is unknown.
Methods: The TRIBE-AKI study was a prospective cohort study of 1594 adults undergoing cardiac surgery at six hospitals between July 2007 and December 2010. We classified the degree of exposure to ACEi/ARB into three categories: 'none' (no exposure prior to surgery), 'held' (on chronic ACEi/ARB but held on the morning of surgery) or 'continued' (on chronic ACEi/ARB and taken the morning of surgery). The co-primary outcomes were 'functional' AKI based upon changes in pre- to postoperative serum creatinine, and 'structural AKI', based upon peak postoperative levels of four urinary biomarkers of kidney injury.
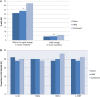
Results: Across the three levels (none, held and continued) of ACEi/ARB exposure there was a graded increase in functional AKI, as defined by AKI stage 1 or worse; (31, 34 and 42%, P for trend 0.03) and by percentage change in serum creatinine from pre- to postoperative (25, 26 and 30%, P for trend 0.03). In contrast, there were no differences in structural AKI across the strata of ACEi/ARB exposure, as assessed by four structural AKI biomarkers (neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, interleukin-18 or liver-fatty acid-binding protein).
Conclusions: Preoperative ACEi/ARB usage was associated with functional but not structural acute kidney injury. As AKI from ACEi/ARB in this setting is unclear, interventional studies testing different strategies of perioperative ACEi/ARB use are warranted.
Keywords: acute renal failure; biomarkers; serum creatinine.
Figures


References
-
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi:10.2215/CJN.00240605. - DOI - PubMed
-
- Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi:10.1053/j.ajkd.2008.11.034. - DOI - PMC - PubMed
-
- Kheterpal S, Khodaparast O, Shanks A, et al. Chronic angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy combined with diuretic therapy is associated with increased episodes of hypotension in noncardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:180–186. doi:10.1053/j.jvca.2007.12.020. - DOI - PubMed
-
- Bertrand M, Godet G, Meersschaert K, et al. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26–30. doi:10.1097/00000539-200101000-00006. - DOI - PubMed
-
- Brabant SM, Bertrand M, Eyraud D, et al. The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists. Anesth Analg. 1999;89:1388–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01DK082185/DK/NIDDK NIH HHS/United States
- R01 HL085757/HL/NHLBI NIH HHS/United States
- K24DK090203/DK/NIDDK NIH HHS/United States
- K23 DK080132/DK/NIDDK NIH HHS/United States
- R01HL085757/HL/NHLBI NIH HHS/United States
- R01 DK096549/DK/NIDDK NIH HHS/United States
- K23 DK081616/DK/NIDDK NIH HHS/United States
- K23DK080132/DK/NIDDK NIH HHS/United States
- K24 DK090203/DK/NIDDK NIH HHS/United States
- P30 DK079310/DK/NIDDK NIH HHS/United States
- L30 DK084760/DK/NIDDK NIH HHS/United States
- R01DK096549/DK/NIDDK NIH HHS/United States
- U01 DK082185/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

