Randomized trial to assess the impact of venlafaxine and soy protein on hot flashes and quality of life in men with prostate cancer
- PMID: 24081940
- PMCID: PMC3816959
- DOI: 10.1200/JCO.2012.48.1432
Randomized trial to assess the impact of venlafaxine and soy protein on hot flashes and quality of life in men with prostate cancer
Abstract
Purpose: Hot flashes occur in approximately 80% of androgen-deprived men. Few intervention studies have been conducted to relieve hot flashes in men.
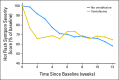
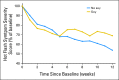
Patients and methods: Eligible androgen-deprived men were randomly assigned to one of four daily regimens (2 × 2 factorial design) for 12 weeks: milk protein powder and placebo pill, venlafaxine and milk protein powder, soy protein powder and placebo pill, or venlafaxine and soy protein powder. The primary end point was hot flash symptom severity score (HFSSS), defined as number of hot flashes times severity. The secondary end point was quality of life (QoL), assessed by using the Functional Assessment of Cancer Therapy-Prostate.
Results: In all, 120 men age 46 to 91 years participated. Most were white (78%) and overweight or obese (83%). Toxicity was minimal. Neither venlafaxine nor soy protein alone or in combination had a significant effect on HFSSS. Soy protein, but not venlafaxine, improved measures of QoL.
Conclusion: In androgen-deprived men, neither venlafaxine nor soy proved effective in reducing hot flashes. Interventions that appear effective for decreasing hot flashes in women may not always turn out to be effective in men.
Trial registration: ClinicalTrials.gov NCT00354432.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Schow DA, Renfer LG, Rozanski TA, et al. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91:855–857. - PubMed
-
- Charig CR, Rundle JS. Flushing: Long-term side effect of orchiectomy in treatment of prostatic carcinoma. Urology. 1989;33:175–178. - PubMed
-
- Quella S, Loprinzi CL, Dose AM. A qualitative approach to defining “hot flashes” in men. Urol Nurs. 1994;14:155–158. - PubMed
-
- Shanafelt TD, Barton DL, Adjei AA, et al. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–1218. - PubMed
-
- Roth AJ, Scher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology. 1998;7:129–132. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

