Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy
- PMID: 24081947
- PMCID: PMC3804937
- DOI: 10.1084/jem.20131219
Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy
Abstract
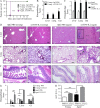
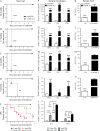
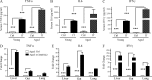
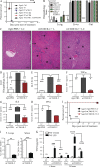
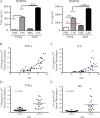
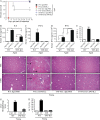
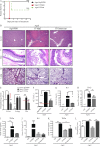
Cancer commonly occurs in the elderly and immunotherapy (IT) is being increasingly applied to this population. However, the majority of preclinical mouse tumor models assessing potential efficacy and toxicities of therapeutics use young mice. We assessed the impact of age on responses to systemic immune stimulation. In contrast to young mice, systemic cancer IT regimens or LPS given to aged mice resulted in rapid and lethal toxicities affecting multiple organs correlating with heightened proinflammatory cytokines systemically and within the parenchymal tissues. This inflammatory response and increased morbidity with age was independent of T cells or NK cells. However, prior in vivo depletion of macrophages in aged mice resulted in lesser cytokine levels, increased survival, and decreased liver histopathology. Furthermore, macrophages from aged mice and normal human elderly volunteers displayed heightened TNF and IL-6 production upon in vitro stimulation. Treatment of both TNF knockout mice and in vivo TNF blockade in aged mice resulted in significant increases in survival and lessened pathology. Importantly, TNF blockade in tumor-bearing, aged mice receiving IT displayed significant anti-tumor effects. These data demonstrate the critical role of macrophages in the age-associated hyper-inflammatory cytokine responses to systemic immunostimulation and underscore the importance of performing preclinical assessments in aged mice.
Figures

Comment in
-
Cancer immunotherapy for the elderly.Nat Rev Immunol. 2013 Nov;13(11):774. doi: 10.1038/nri3557. Epub 2013 Oct 11. Nat Rev Immunol. 2013. PMID: 24113867 No abstract available.
-
Real-world illness requires medical multitasking.Nature. 2018 May;557(7703):5-6. doi: 10.1038/d41586-018-05028-w. Nature. 2018. PMID: 29717257 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

