Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop
- PMID: 24081990
- PMCID: PMC3818355
- DOI: 10.4049/jimmunol.1300354
Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop
Abstract
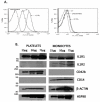
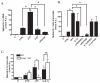
LPS activates platelets through TLR4, aiding productive sepsis, with stimulated splicing and translation of stored heteronuclear pro-IL-1β RNA. Although the IL-1R type 1 (IL-1R1) receptor for IL-1 shares downstream components with the TLR4 receptor, platelets are not known to express IL-1R1, nor are they known to respond to this cytokine. We show by flow cytometry and Western blotting that platelets express IL-1R1, and that IL-1β and IL-1α stimulate heteronuclear I-1β splicing and translation of the newly made mRNA in platelets. Platelets also respond to the IL-1β they make, which is exclusively associated with shed microparticles. Specific blockade of IL-1R1 with IL-1R antagonist suppressed platelet stimulation by IL-1, so IL-1β stimulates its own synthesis in an autocrine signaling loop. Strikingly, IL-1R antagonist inhibition, pharmacologic or genetic suppression of pro-IL-1β processing to active cytokine by caspase-1, or blockade of de novo protein synthesis also blocked LPS-induced IL-1β mRNA production. Robust stimulation of platelets by LPS therefore also required IL-1β amplification. Activated platelets made IL-1β in vivo as IL-1β rapidly accumulated in occluded murine carotid arteries by posttranscriptional RNA splicing unique to platelets. We conclude that IL-1β is a platelet agonist, that IL-1β acts through an autocrine stimulatory loop, that an IL-1β autocrine loop is required to amplify platelet activation by LPS, and that platelets immobilized in occlusive thrombi are activated over time to produce IL-1β. IL-1 is a new platelet agonist that promotes its own synthesis, connecting thrombosis with immunity.
Figures
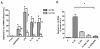
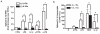


References
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. - PubMed
-
- Dinarello CA. Interleukin-1beta and the autoinflammatory diseases. N. Engl. J. Med. 2009;360:2467–2470. - PubMed
-
- Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 2007;179:1913–1925. - PubMed
-
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

