Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells
- PMID: 24082012
- PMCID: PMC3896662
- DOI: 10.1189/jlb.0513297
Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells
Abstract
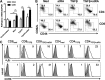
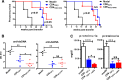
It is known that ATRA promotes the development of TGF-β-induced CD4(+)Foxp3(+) iTregs, which play a vital role in the prevention of autoimmune diseases; however, the role of ATRA in facilitating the differentiation and function of CD8(+)Foxp3(+) iTregs remains elusive. Using a head-to-head comparison, we found that ATRA promoted expression of Foxp3 and development of CD4(+) iTregs, but it did not promote Foxp3 expression on CD8(+) cells. Using a standard in vitro assay, we demonstrated that CD8(+) iTregs induced by TGF-β and ATRA were not superior to CD8(+) iTregs induced by TGF-β alone. In cGVHD, in a typical lupus syndrome model where DBA2 spleen cells were transferred to DBA2xC57BL/6 F1 mice, we observed that both CD8(+) iTregs induced by TGF-β and ATRA and those induced by TGF-β alone had similar therapeutic effects. ATRA did not boost but, conversely, impaired the differentiation and function of human CD8(+) iTregs. CD8(+) cells expressed the ATRA receptor RAR and responded to ATRA, similar to CD4(+) cells. We have identified the differential role of ATRA in promoting Foxp3(+) Tregs in CD4(+) and CD8(+) cell populations. These results will help to determine a protocol for developing different Treg cell populations and may provide novel insights into clinical cell therapy for patients with autoimmune diseases and those needing organ transplantation.
Keywords: Autoimmunity; Foxp3; GVHD; TGF-β; all-trans retinoic acid; regulatory T cells.
Figures



References
-
- Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 - PubMed
-
- Horwitz D. A., Zheng S. G., Gray J. D. (2008) Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 29, 429–435 - PubMed
-
- Cosmi L., Liotta F., Lazzeri E., Francalanci M., Angeli R., Mazzinghi B., Santarlasci V., Manetti R., Vanini V., Romagnani P., Maggi E., Romagnani S., Annunziato F. (2003) Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood 102, 4107–4114 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

