CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L
- PMID: 24082013
- PMCID: PMC3896656
- DOI: 10.1189/jlb.0113013
CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L
Abstract
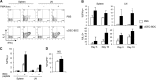
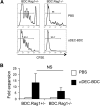
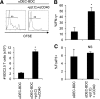
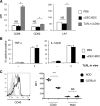
DCs are important mediators of peripheral tolerance for the prevention of autoimmunity. Chimeric αDEC-205 antibodies with attached antigens allow in vivo antigen-specific stimulation of T cells by CD8(+) DCs, resulting in tolerance in nonautoimmune mice. However, it is not clear whether DC-mediated tolerance induction occurs in the context of ongoing autoimmunity. We assessed the role of CD8(+) DCs in stimulation of autoreactive CD4(+) T cells in the NOD mouse model of type 1 diabetes. Targeting of antigen to CD8(+) DCs via αDEC-205 led to proliferation and expansion of β-cell specific BDC2.5 T cells. These T cells also produced IL-2 and IFN-γ and did not up-regulate FoxP3, consistent with an activated rather than tolerant phenotype. Similarly, endogenous BDC peptide-reactive T cells, identified with I-A(g7) tetramers, did not become tolerant after antigen delivery via αDEC-205: no deletion or Treg induction was observed. We observed that CD8(+) DCs from NOD mice expressed higher surface levels of CD40 than CD8(+) DCs from C57BL/6 mice. Blockade of CD40-CD40L interactions reduced the number of BDC2.5 T cells remaining in mice, 10 days after antigen targeting to CD8 DCs, and blocked IFN-γ production by BDC2.5 T cells. These data indicate that the ability of autoreactive CD4(+) T cells to undergo tolerance mediated by CD8(+) DCs is defective in NOD mice and that blocking CD40-CD40L interactions can restore tolerance induction.
Keywords: CD40; DEC-205; peripheral tolerance; type 1 diabetes.
Figures



Similar articles
-
DCIR2+ cDC2 DCs and Zbtb32 Restore CD4+ T-Cell Tolerance and Inhibit Diabetes.Diabetes. 2015 Oct;64(10):3521-31. doi: 10.2337/db14-1880. Epub 2015 Jun 12. Diabetes. 2015. PMID: 26070317 Free PMC article.
-
Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice.Proc Natl Acad Sci U S A. 2008 Apr 29;105(17):6374-9. doi: 10.1073/pnas.0802644105. Epub 2008 Apr 22. Proc Natl Acad Sci U S A. 2008. PMID: 18430797 Free PMC article.
-
CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T cell-dependent, licensed CD8+ T cell responses.J Leukoc Biol. 2010 Mar;87(3):477-85. doi: 10.1189/jlb.0809535. Epub 2009 Dec 1. J Leukoc Biol. 2010. PMID: 19952354 Free PMC article.
-
Breaking T cell tolerance to beta cell antigens by merocytic dendritic cells.Cell Mol Life Sci. 2011 Sep;68(17):2873-83. doi: 10.1007/s00018-011-0730-6. Epub 2011 May 31. Cell Mol Life Sci. 2011. PMID: 21626409 Free PMC article. Review.
-
Aspirin and the induction of tolerance by dendritic cells.Handb Exp Pharmacol. 2009;(188):197-213. doi: 10.1007/978-3-540-71029-5_9. Handb Exp Pharmacol. 2009. PMID: 19031027 Review.
Cited by
-
Soluble RAGE Prevents Type 1 Diabetes Expanding Functional Regulatory T Cells.Diabetes. 2022 Sep 1;71(9):1994-2008. doi: 10.2337/db22-0177. Diabetes. 2022. PMID: 35713929 Free PMC article.
-
Gaps in Aging Research as it Applies to Rheumatologic Clinical Care.Clin Geriatr Med. 2017 Feb;33(1):119-133. doi: 10.1016/j.cger.2016.08.009. Epub 2016 Oct 18. Clin Geriatr Med. 2017. PMID: 27886693 Free PMC article. Review.
-
Cellular immunological changes in patients with LADA are a mixture of those seen in patients with type 1 and type 2 diabetes.Clin Exp Immunol. 2019 Jul;197(1):64-73. doi: 10.1111/cei.13289. Epub 2019 Mar 24. Clin Exp Immunol. 2019. PMID: 30843600 Free PMC article.
-
The Role of Dendritic Cell Subsets and Innate Immunity in the Pathogenesis of Type 1 Diabetes and Other Autoimmune Diseases.Front Immunol. 2015 Jun 15;6:288. doi: 10.3389/fimmu.2015.00288. eCollection 2015. Front Immunol. 2015. PMID: 26124756 Free PMC article. Review.
-
Altered Function of Antigen-Presenting Cells in Type 1 Diabetes: A Challenge for Antigen-Specific Immunotherapy?Diabetes. 2018 Aug;67(8):1481-1494. doi: 10.2337/db17-1564. Diabetes. 2018. PMID: 30030289 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

