Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth
- PMID: 24082114
- PMCID: PMC3801037
- DOI: 10.1073/pnas.1313650110
Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth
Abstract
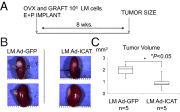
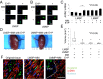
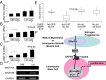
Uterine leiomyomas are extremely common estrogen and progesterone-dependent tumors of the myometrium and cause irregular uterine bleeding, severe anemia, and recurrent pregnancy loss in 15-30% of reproductive-age women. Each leiomyoma is thought to arise from a single mutated myometrial smooth muscle stem cell. Leiomyoma side-population (LMSP) cells comprising 1% of all tumor cells and displaying tumor-initiating stem cell characteristics are essential for estrogen- and progesterone-dependent in vivo growth of tumors, although they have remarkably lower estrogen/progesterone receptor levels than mature myometrial or leiomyoma cells. However, how estrogen/progesterone regulates the growth of LMSP cells via mature neighboring cells is unknown. Here, we demonstrate a critical paracrine role of the wingless-type (WNT)/β-catenin pathway in estrogen/progesterone-dependent tumorigenesis, involving LMSP and differentiated myometrial or leiomyoma cells. Estrogen/progesterone treatment of mature myometrial cells induced expression of WNT11 and WNT16, which remained constitutively elevated in leiomyoma tissues. In LMSP cells cocultured with mature myometrial cells, estrogen-progesterone selectively induced nuclear translocation of β-catenin and induced transcriptional activity of its heterodimeric partner T-cell factor and their target gene AXIN2, leading to the proliferation of LMSP cells. This effect could be blocked by a WNT antagonist. Ectopic expression of inhibitor of β-catenin and T-cell factor 4 in LMSP cells, but not in mature leiomyoma cells, blocked the estrogen/progesterone-dependent growth of human tumors in vivo. We uncovered a paracrine role of the WNT/β-catenin pathway that enables mature myometrial or leiomyoma cells to send mitogenic signals to neighboring tissue stem cells in response to estrogen and progesterone, leading to the growth of uterine leiomyomas.
Keywords: WNT/β-catenin signaling; paracrine signaling; tumor biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Walker CL, Stewart EA. Uterine fibroids: The elephant in the room. Science. 2005;308(5728):1589–1592. - PubMed
-
- Wallach EE, Vlahos NF. Uterine myomas: An overview of development, clinical features, and management. Obstet Gynecol. 2004;104(2):393–406. - PubMed
-
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571–588. - PubMed
-
- Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. - PubMed
-
- Szotek PP, et al. Adult mouse myometrial label-retaining cells divide in response to gonadotropin stimulation. Stem Cells. 2007;25(5):1317–1325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

