Computational study of the activated O(H) state in the catalytic mechanism of cytochrome c oxidase
- PMID: 24082138
- PMCID: PMC3801011
- DOI: 10.1073/pnas.1220379110
Computational study of the activated O(H) state in the catalytic mechanism of cytochrome c oxidase
Abstract
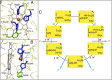
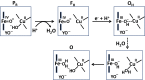
Complex IV in the respiratory chain of mitochondria and bacteria catalyzes reduction of molecular oxygen to water, and conserves much of the liberated free energy as an electrochemical proton gradient, which is used for the synthesis of ATP. Photochemical electron injection experiments have shown that reduction of the ferric/cupric state of the enzyme's binuclear heme a3/CuB center is coupled to proton pumping across the membrane, but only if oxidation of the reduced enzyme by O2 immediately precedes electron injection. In contrast, reduction of the binuclear center in the "as-isolated" ferric/cupric enzyme is sluggish and without linkage to proton translocation. During turnover, the binuclear center apparently shuttles via a metastable but activated ferric/cupric state (O(H)), which may decay into a more stable catalytically incompetent form (O) in the absence of electron donors. The structural basis for the difference between these two states has remained elusive, and is addressed here using computational methodology. The results support the notion that CuB[II] is either three-coordinated in the O(H) state or shares an OH(-) ligand with heme a3 in a strained μ-hydroxo structure. Relaxation to state O is initiated by hydration of the binuclear site. The redox potential of CuB is expected, and found by density functional theory calculations, to be substantially higher in the O(H) state than in state O. Our calculations also suggest that the neutral radical form of the cross-linked tyrosine in the binuclear site may be more significant in the catalytic cycle than suspected so far.
Keywords: electron transfer; oxygen reduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ferguson-Miller S, Babcock GT. Heme-copper terminal oxidases. Chem Rev. 1996;96(7):2889–2908. - PubMed
-
- Kaila VRI, Verkhovsky MI, Wikström M. Proton-coupled electron transfer in cytochrome oxidase. Chem Rev. 2010;110(12):7062–7081. - PubMed
-
- Yoshikawa S, et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280(5370):1723–1729. - PubMed
-
- Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376(6542):660–669. - PubMed
-
- Konstantinov AA, Siletsky S, Mitchell D, Kaulen A, Gennis RB. The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer. Proc Natl Acad Sci USA. 1997;94(17):9085–9090. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

