Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice
- PMID: 24083388
- PMCID: PMC3851031
- DOI: 10.1186/2040-2392-4-35
Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice
Abstract
Background: Previously, we identified multiple, rare serotonin (5-HT) transporter (SERT) variants in children with autism spectrum disorder (ASD). Although in our study the SERT Ala56 variant was over-transmitted to ASD probands, it was also seen in some unaffected individuals, suggesting that associated ASD risk is influenced by the epistatic effects of other genetic variation. Subsequently, we established that mice expressing the SERT Ala56 variant on a 129S6/S4 genetic background display multiple biochemical, physiological and behavioral changes, including hyperserotonemia, altered 5-HT receptor sensitivity, and altered social, communication, and repetitive behavior. Here we explore the effects of genetic background on SERT Ala56 knock-in phenotypes.
Methods: To explore the effects of genetic background, we backcrossed SERT Ala56 mice on the 129 background into a C57BL/6 (B6) background to achieve congenic B6 SERT Ala56 mice, and assessed autism-relevant behavior, including sociability, ultrasonic vocalizations, and repetitive behavior in the home cage, as well as serotonergic phenotypes, including whole blood serotonin levels and serotonin receptor sensitivity.
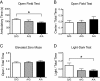
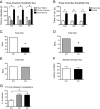
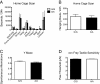
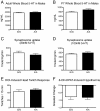
Results: One consistent phenotype between the two strains was performance in the tube test for dominance, where mutant mice displayed a greater tendency to withdraw from a social encounter in a narrow tube as compared to wildtype littermate controls. On the B6 background, mutant pup ultrasonic vocalizations were significantly increased, in contrast to decreased vocalizations seen previously on the 129 background. Several phenotypes seen on the 129 background were reduced or absent when the mutation was placed on the B6 background, including hyperserotonemia, 5-HT receptor hypersensivity, and repetitive behavior.
Conclusions: Our findings provide a cogent example of how epistatic interactions can modulate the impact of functional genetic variation and suggest that some aspects of social behavior may be especially sensitive to changes in SERT function. Finally, these results provide a platform for the identification of genes that may modulate the risk of ASD in humans.
Figures
References
-
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. - DOI - PubMed
-
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

