Slow DNA transport through nanopores in hafnium oxide membranes
- PMID: 24083444
- PMCID: PMC4729694
- DOI: 10.1021/nn404326f
Slow DNA transport through nanopores in hafnium oxide membranes
Abstract
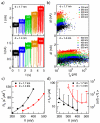
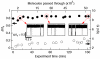
We present a study of double- and single-stranded DNA transport through nanopores fabricated in ultrathin (2-7 nm thick) freestanding hafnium oxide (HfO2) membranes. The high chemical stability of ultrathin HfO2 enables long-lived experiments with <2 nm diameter pores that last several hours, in which we observe >50 000 DNA translocations with no detectable pore expansion. Mean DNA velocities are slower than velocities through comparable silicon nitride pores, providing evidence that HfO2 nanopores have favorable physicochemical interactions with nucleic acids that can be leveraged to slow down DNA in a nanopore.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

