Vaccines in development against West Nile virus
- PMID: 24084235
- PMCID: PMC3814594
- DOI: 10.3390/v5102384
Vaccines in development against West Nile virus
Abstract
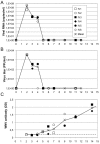
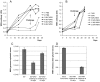
West Nile encephalitis emerged in 1999 in the United States, then rapidly spread through the North American continent causing severe disease in human and horses. Since then, outbreaks appeared in Europe, and in 2012, the United States experienced a new severe outbreak reporting a total of 5,387 cases of West Nile virus (WNV) disease in humans, including 243 deaths. So far, no human vaccine is available to control new WNV outbreaks and to avoid worldwide spreading. In this review, we discuss the state-of-the-art of West Nile vaccine development and the potential of a novel safe and effective approach based on recombinant live attenuated measles virus (MV) vaccine. MV vaccine is a live attenuated negative-stranded RNA virus proven as one of the safest, most stable and effective human vaccines. We previously described a vector derived from the Schwarz MV vaccine strain that stably expresses antigens from emerging arboviruses, such as dengue, West Nile or chikungunya viruses, and is strongly immunogenic in animal models, even in the presence of MV pre-existing immunity. A single administration of a recombinant MV vaccine expressing the secreted form of WNV envelope glycoprotein elicited protective immunity in mice and non-human primates as early as two weeks after immunization, indicating its potential as a human vaccine.
Figures

References
-
- Smithburn K.C.H., Burke A.W., Paul J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940;20:471–472.
-
- Zeller H.G., Schuffenecker I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2004;23:147–156. doi: 10.1007/s10096-003-1085-1. - DOI - PubMed
-
- Prevention, Centers for Disease Control and Prevention (CDC) West Nile Virus (WNV) Human Infections Reported to ArboNET, by State, United States, 2012 (as of December 11, 2012). 2012 [cited 2012 21–02–2013] [(accessed on 6 October 2013)]. Available online: http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detaile....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

