Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials
- PMID: 24084496
- PMCID: PMC3892560
- DOI: 10.1016/j.ophtha.2013.08.015
Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials
Abstract
Purpose: To describe risk factors for geographic atrophy (GA) in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT).
Design: Cohort within a randomized clinical trial.
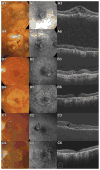
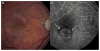
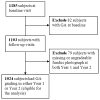
Participants: We analyzed 1024 CATT patients with no GA visible on color fundus photographs (CFPs) and/or fluorescein angiograms (FAs) at enrollment.
Methods: Eyes were assigned to ranibizumab (0.5 mg) or bevacizumab (1.25 mg) treatment and to a 2-year monthly or pro re nata (PRN) injection regimen, or monthly injections for 1 year and PRN for 1 year. Demographic, genetic, and baseline ocular characteristics and lesion features of CFP/FA and optical coherence tomography (OCT) were evaluated as risk factors for GA through 2 years of follow-up. Time-dependent Cox proportional hazard models were used to estimate adjusted hazard ratios (aHRs).
Main outcome measures: Development of GA.
Results: By 2 years, GA developed in 187 of 1024 patients (18.3%). Baseline risk factors for GA development included baseline visual acuity (VA) ≤20/200 (aHR, 2.65; 95% confidence interval [CI], 1.43-4.93), retinal angiomatous proliferation (RAP; aHR, 1.69; 95% CI, 1.16-2.47), GA in the fellow eye (aHR, 2.07; 95% CI, 1.40-3.08), and intraretinal fluid at the foveal center (aHR, 2.10; 95% CI, 1.34-3.31). Baseline factors associated with lower risk for GA development included blocked fluorescence (aHR, 0.49; 95% CI, 0.29-0.82), OCT measurements of subretinal fluid thickness of >25 μ (aHR, 0.52; 95% CI, 0.35-0.78), subretinal tissue complex thickness of >275 compared with ≤75 μ (aHR, 0.31; 95% CI, 0.19-0.50), and vitreomacular attachment (aHR, 0.55; 95% CI, 0.31-0.97). Ranibizumab compared with bevacizumab had a higher risk (aHR, 1.43; 95% CI, 1.06-1.93), and monthly dosing had a higher risk (aHR, 1.59; 95% CI, 1.17-2.16) than PRN dosing. There were no strong associations between development of GA and the presence of risk alleles for CFH, ARMS 2, HTRA1, C3, or TLR3.
Conclusions: Approximately one fifth of CATT patients developed GA within 2 years of treatment. Independent baseline risk factors included poor VA, RAP, foveal intraretinal fluid, monthly dosing, and treatment with ranibizumab. Anti-vascular endothelial growth factor therapy may have a role in the development of GA.
Copyright © 2014 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Re: Grunwald et al.: risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials (ophthalmology 2014;121:150-61).Ophthalmology. 2014 Jul;121(7):e34. doi: 10.1016/j.ophtha.2013.12.043. Epub 2014 Mar 7. Ophthalmology. 2014. PMID: 24613823 No abstract available.
-
Author reply: To PMID 24084496.Ophthalmology. 2014 Jul;121(7):e35. doi: 10.1016/j.ophtha.2014.01.023. Epub 2014 Mar 7. Ophthalmology. 2014. PMID: 24613824 No abstract available.
References
-
- Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. - PubMed
-
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. - PubMed
-
- Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

