Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies
- PMID: 24084736
- PMCID: PMC3809806
- DOI: 10.1172/JCI71543
Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies
Abstract
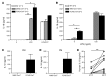
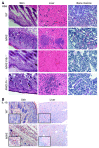
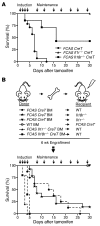
The inflammasome is a cytoplasmic multiprotein complex that promotes proinflammatory cytokine maturation in response to host- and pathogen-derived signals. Missense mutations in cryopyrin (NLRP3) result in a hyperactive inflammasome that drives overproduction of the proinflammatory cytokines IL-1β and IL-18, leading to the cryopyrin-associated periodic syndromes (CAPS) disease spectrum. Mouse lines harboring CAPS-associated mutations in Nlrp3 have elevated levels of IL-1β and IL-18 and closely mimic human disease. To examine the role of inflammasome-driven IL-18 in murine CAPS, we bred Nlrp3 mutations onto an Il18r-null background. Deletion of Il18r resulted in partial phenotypic rescue that abolished skin and visceral disease in young mice and normalized serum cytokines to a greater extent than breeding to Il1r-null mice. Significant systemic inflammation developed in aging Nlrp3 mutant Il18r-null mice, indicating that IL-1 and IL-18 drive pathology at different stages of the disease process. Ongoing inflammation in double-cytokine knockout CAPS mice implicated a role for caspase-1-mediated pyroptosis and confirmed that CAPS is inflammasome dependent. Our results have important implications for patients with CAPS and residual disease, emphasizing the need to explore other NLRP3-mediated pathways and the potential for inflammasome-targeted therapy.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

