FGF19 action in the brain induces insulin-independent glucose lowering
- PMID: 24084738
- PMCID: PMC3809800
- DOI: 10.1172/JCI70710
FGF19 action in the brain induces insulin-independent glucose lowering
Abstract
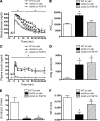
Insulin-independent glucose disposal (referred to as glucose effectiveness [GE]) is crucial for glucose homeostasis and, until recently, was thought to be invariable. However, GE is reduced in type 2 diabetes and markedly decreased in leptin-deficient ob/ob mice. Strategies aimed at increasing GE should therefore be capable of improving glucose tolerance in these animals. The gut-derived hormone FGF19 has previously been shown to exert potent antidiabetic effects in ob/ob mice. In ob/ob mice, we found that systemic FGF19 administration improved glucose tolerance through its action in the brain and that a single, low-dose i.c.v. injection of FGF19 dramatically improved glucose intolerance within 2 hours. Minimal model analysis of glucose and insulin data obtained during a frequently sampled i.v. glucose tolerance test showed that the antidiabetic effect of i.c.v. FGF19 was solely due to increased GE and not to changes of either insulin secretion or insulin sensitivity. The mechanism underlying this effect appears to involve increased metabolism of glucose to lactate. Together, these findings implicate the brain in the antidiabetic action of systemic FGF19 and establish the brain’s capacity to rapidly, potently, and selectively increase insulin-independent glucose disposal.
Figures





References
-
- Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19(9):1018–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007247/DK/NIDDK NIH HHS/United States
- DK017047/DK/NIDDK NIH HHS/United States
- R01 DK089056/DK/NIDDK NIH HHS/United States
- R01 DK083042/DK/NIDDK NIH HHS/United States
- DK083042/DK/NIDDK NIH HHS/United States
- DK035816/DK/NIDDK NIH HHS/United States
- F32 DK097859/DK/NIDDK NIH HHS/United States
- R01 DK090320/DK/NIDDK NIH HHS/United States
- DK089053/DK/NIDDK NIH HHS/United States
- R01 DK029867/DK/NIDDK NIH HHS/United States
- P30 DK035816/DK/NIDDK NIH HHS/United States
- DK090320/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

