Antimicrobial peptides from marine proteobacteria
- PMID: 24084784
- PMCID: PMC3826127
- DOI: 10.3390/md11103632
Antimicrobial peptides from marine proteobacteria
Abstract
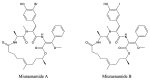
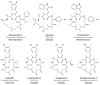
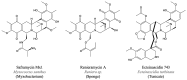
After years of inadequate use and the emergence of multidrug resistant (MDR) strains, the efficiency of "classical" antibiotics has decreased significantly. New drugs to fight MDR strains are urgently needed. Bacteria hold much promise as a source of unusual bioactive metabolites. However, the potential of marine bacteria, except for Actinomycetes and Cyanobacteria, has been largely underexplored. In the past two decades, the structures of several antimicrobial compounds have been elucidated in marine Proteobacteria. Of these compounds, polyketides (PKs), synthesised by condensation of malonyl-coenzyme A and/or acetyl-coenzyme A, and non-ribosomal peptides (NRPs), obtained through the linkage of (unusual) amino acids, have recently generated particular interest. NRPs are good examples of naturally modified peptides. Here, we review and compile the data on the antimicrobial peptides isolated from marine Proteobacteria, especially NRPs.
Figures
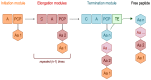
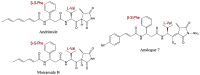
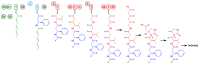

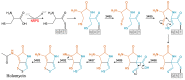


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

