Brain regulation of thrombosis and hemostasis: from theory to practice
- PMID: 24085025
- PMCID: PMC3954774
- DOI: 10.1161/STROKEAHA.113.000736
Brain regulation of thrombosis and hemostasis: from theory to practice
Keywords: blood-brain barrier; hemorrhage; hemostasis; thrombosis.
Figures
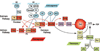
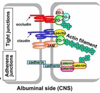
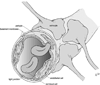
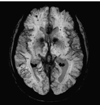

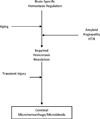
References
-
- Aird WC. Coagulation. Crit Care Med. 2005;33(Suppl):S485–S487. - PubMed
-
- Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–2281. - PubMed
-
- Rosenberg RD, Aird WC. Vascular-bed-specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–1564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

