Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid
- PMID: 24085744
- PMCID: PMC3826687
- DOI: 10.1194/jlr.M042051
Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid
Abstract
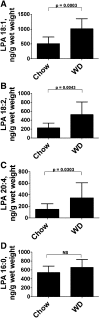
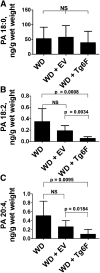
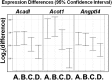
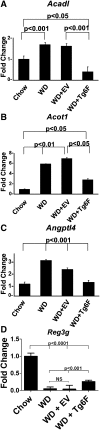
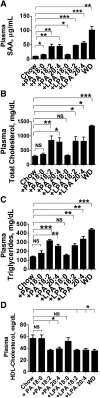
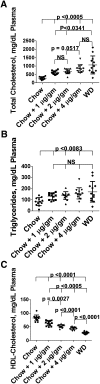
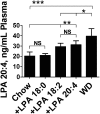
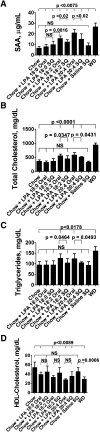
We recently reported that levels of unsaturated lysophosphatidic acid (LPA) in the small intestine significantly correlated with the extent of aortic atherosclerosis in LDL receptor-null (LDLR⁻/⁻) mice fed a Western diet (WD). Here we demonstrate that WD increases unsaturated (but not saturated) LPA levels in the small intestine of LDLR⁻/⁻ mice and causes changes in small intestine gene expression. Confirmation of microarray analysis by quantitative RT-PCR showed that adding transgenic tomatoes expressing the apoA-I mimetic peptide 6F (Tg6F) to WD prevented many WD-mediated small intestine changes in gene expression. If instead of feeding WD, unsaturated LPA was added to chow and fed to the mice: i) levels of LPA in the small intestine were similar to those induced by feeding WD; ii) gene expression changes in the small intestine mimicked WD-mediated changes; and iii) changes in plasma serum amyloid A, total cholesterol, triglycerides, HDL-cholesterol levels, and the fast-performance liquid chromatography lipoprotein profile mimicked WD-mediated changes. Adding Tg6F (but not control tomatoes) to LPA-supplemented chow prevented the LPA-induced changes. We conclude that: i) WD-mediated systemic inflammation and dyslipidemia may be in part due to WD-induced increases in small intestine LPA levels; and ii) Tg6F reduces WD-mediated systemic inflammation and dyslipidemia by preventing WD-induced increases in LPA levels in the small intestine.
Keywords: 6F peptide; apolipoprotein A-I mimetic peptides; atherosclerosis; genetically engineered tomato plants; lysophosphatidic acid.
Figures





Comment in
-
Tomatoes, lysophosphatidic acid, and the small intestine: new pieces in the puzzle of apolipoprotein mimetic peptides?J Lipid Res. 2013 Dec;54(12):3223-6. doi: 10.1194/jlr.E045054. Epub 2013 Oct 21. J Lipid Res. 2013. PMID: 24146211 Free PMC article. No abstract available.
References
-
- Navab M., Reddy S. T., Van Lenten B. J., Buga G. M., Hough G., Wagner A. C., Fogelman A. M. 2012. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: novel hypotheses and review of literature. Arterioscler. Thromb. Vasc. Biol. 32: 2553–2560 - PMC - PubMed
-
- Watson C. E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S. T., et al. 2011. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 52: 361–373 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

