Melanocortin 4 receptor becomes an ACTH receptor by coexpression of melanocortin receptor accessory protein 2
- PMID: 24085819
- PMCID: PMC5427830
- DOI: 10.1210/me.2013-1099
Melanocortin 4 receptor becomes an ACTH receptor by coexpression of melanocortin receptor accessory protein 2
Abstract
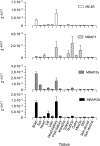
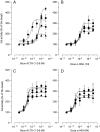
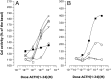
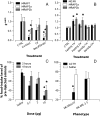
Melanocortin 2 receptor (MC2R) is the only canonical ACTH receptor. Its functional expression requires the presence of an accessory protein, known as melanocortin receptor 2 accessory protein 1 (MRAP1). The vertebrate genome exhibits a paralogue gene called MRAP2, which is duplicated in zebrafish (MRAP2a and MRAP2b), although its function remains unknown. In this paper, we demonstrate that MRAP2a enables MC4R, a canonical MSH receptor, to be activated by ACTH with a similar sensitivity to that exhibited by MC2R. Both proteins physically interact and are coexpressed in the neurons of the preoptic area, a key region in the control of the energy balance and hypophyseal secretion in fish. ACTH injections inhibit food intake in wild-type zebrafish but not in fish lacking functional MC4R. Both MRAP1 and MRAP2a are hormonally regulated, suggesting that these proteins are substrates for feed-back regulatory pathways of melanocortin signaling. Fasting has no effect on the central expression of MRAP2a but stimulates MRAP2b expression. This protein interacts and is colocalized with MC4R in the tuberal hypothalamic neurons but has no effect on the pharmacologic profile of MC4R. However, MRPA2b is able to decrease basal reporter activity in cell lines expressing MC4R. It is plausible that MRAP2b decreases the constitutive activity of the MC4R during fasting periods, driving the animal toward a positive energy balance. Our data indicate that MRAP2s control the activity of MC4R, opening up new pathways for the regulation of melanocortin signaling and, by extension, for the regulation of the energy balance and obesity.
Figures





References
-
- Nakanishi S, Inoue A, Kita T, et al. . Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature. 1979;278:423–427. - PubMed
-
- Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57. - PubMed
-
- Schiöth HB, Haitina T, Ling MK, et al. . Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides. 2005;26:1886–1900. - PubMed
-
- Chan LF, Metherell LA, Clark AJ. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol. 2011;660:171–180. - PubMed
-
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

