Receptor tyrosine kinases in the nucleus
- PMID: 24086039
- PMCID: PMC3783051
- DOI: 10.1101/cshperspect.a008979
Receptor tyrosine kinases in the nucleus
Abstract
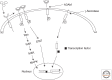
To date, 18 distinct receptor tyrosine kinases (RTKs) are reported to be trafficked from the cell surface to the nucleus in response to ligand binding or heterologous agonist exposure. In most cases, an intracellular domain (ICD) fragment of the receptor is generated at the cell surface and translocated to the nucleus, whereas for a few others the intact receptor is translocated to the nucleus. ICD fragments are generated by several mechanisms, including proteolysis, internal translation initiation, and messenger RNA (mRNA) splicing. The most prevalent mechanism is intramembrane cleavage by γ-secretase. In some cases, more than one mechanism has been reported for the nuclear localization of a specific RTK. The generation and use of RTK ICD fragments to directly communicate with the nucleus and influence gene expression parallels the production of ICD fragments by a number of non-RTK cell-surface molecules that also influence cell proliferation. This review will be focused on the individual RTKs and to a lesser extent on other growth-related cell-surface transmembrane proteins.
Figures

References
-
- Allison JG, Das PM, Ma J, Inglis FM, Jones FE 2011. The ERBB4 intracellular domain (4ICD) regulates NRG1-induced gene expression in hippocampal neurons. Neurosci Res 70: 155–163 - PubMed
-
- Ancot F, Foveau B, Lefebvre J, Leroy C, Tulasne D 2009. Proteolytic cleavages give receptor tyrosine kinases the gift of ubiquity. Oncogene 28: 2185–2195 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
