Neuroblastoma and MYCN
- PMID: 24086065
- PMCID: PMC3784814
- DOI: 10.1101/cshperspect.a014415
Neuroblastoma and MYCN
Abstract
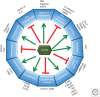
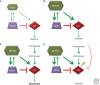
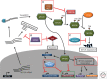
Neuroblastoma, the most common extracranial solid tumor of childhood, is thought to originate from undifferentiated neural crest cells. Amplification of the MYC family member, MYCN, is found in ∼25% of cases and correlates with high-risk disease and poor prognosis. Currently, amplification of MYCN remains the best-characterized genetic marker of risk in neuroblastoma. This article reviews roles for MYCN in neuroblastoma and highlights recent identification of other driver mutations. Strategies to target MYCN at the level of protein stability and transcription are also reviewed.
Figures

References
-
- Akter J, Takatori A, Hossain MS, Ozaki T, Nakazawa A, Ohira M, Suenaga Y, Nakagawara A 2011. Expression of NLRR3 orphan receptor gene is negatively regulated by MYCN and Miz-1, and its downregulation is associated with unfavorable outcome in neuroblastoma. Clin Cancer Res 17: 6681–6692 - PubMed
-
- Arregui CO, Balsamo J, Lilien J 2000. Regulation of signaling by protein-tyrosine phosphatases: Potential roles in the nervous system. Neurochem Res 25: 95–105 - PubMed
-
- Attiyeh EF, London WB, Mossé YP, Wang Q, Winter C, Khazi D, McGrady PW, Seeger RC, Look AT, Shimada H, et al. 2005. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med 353: 2243–2253 - PubMed
-
- Au SL-K, Wong CC-L, Lee JM-F, Fan DN-Y, Tsang FH, Ng IO-L, Wong C-M 2012. Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis. Hepatology 56: 622–631 - PubMed
-
- Banelli B, Gelvi I, Di Vinci A, Scaruffi P, Casciano I, Allemanni G, Bonassi S, Tonini GP, Romani M 2005. Distinct CpG methylation profiles characterize different clinical groups of neuroblastic tumors. Oncogene 24: 5619–5628 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA163155/CA/NCI NIH HHS/United States
- R01 CA102321/CA/NCI NIH HHS/United States
- R01 CA133091/CA/NCI NIH HHS/United States
- P01 CA081403/CA/NCI NIH HHS/United States
- R01 CA159859/CA/NCI NIH HHS/United States
- CA163155/CA/NCI NIH HHS/United States
- CA148699/CA/NCI NIH HHS/United States
- CA102321/CA/NCI NIH HHS/United States
- CA081403/CA/NCI NIH HHS/United States
- R01 CA148699/CA/NCI NIH HHS/United States
- CA133091/CA/NCI NIH HHS/United States
- T32 CA128583/CA/NCI NIH HHS/United States
- U01 CA176287/CA/NCI NIH HHS/United States
- CA159859/CA/NCI NIH HHS/United States
- CA128583/CA/NCI NIH HHS/United States
- T32 CA151022/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
