Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites
- PMID: 24086113
- PMCID: PMC3782411
- DOI: 10.1371/journal.pmed.1001517
Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites
Abstract
Background: Vaccine-serotype (VT) invasive pneumococcal disease (IPD) rates declined substantially following introduction of 7-valent pneumococcal conjugate vaccine (PCV7) into national immunization programs. Increases in non-vaccine-serotype (NVT) IPD rates occurred in some sites, presumably representing serotype replacement. We used a standardized approach to describe serotype-specific IPD changes among multiple sites after PCV7 introduction.
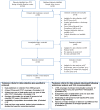
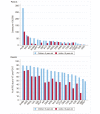
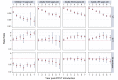
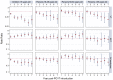
Methods and findings: Of 32 IPD surveillance datasets received, we identified 21 eligible databases with rate data ≥ 2 years before and ≥ 1 year after PCV7 introduction. Expected annual rates of IPD absent PCV7 introduction were estimated by extrapolation using either Poisson regression modeling of pre-PCV7 rates or averaging pre-PCV7 rates. To estimate whether changes in rates had occurred following PCV7 introduction, we calculated site specific rate ratios by dividing observed by expected IPD rates for each post-PCV7 year. We calculated summary rate ratios (RRs) using random effects meta-analysis. For children <5 years old, overall IPD decreased by year 1 post-PCV7 (RR 0.55, 95% CI 0.46-0.65) and remained relatively stable through year 7 (RR 0.49, 95% CI 0.35-0.68). Point estimates for VT IPD decreased annually through year 7 (RR 0.03, 95% CI 0.01-0.10), while NVT IPD increased (year 7 RR 2.81, 95% CI 2.12-3.71). Among adults, decreases in overall IPD also occurred but were smaller and more variable by site than among children. At year 7 after introduction, significant reductions were observed (18-49 year-olds [RR 0.52, 95% CI 0.29-0.91], 50-64 year-olds [RR 0.84, 95% CI 0.77-0.93], and ≥ 65 year-olds [RR 0.74, 95% CI 0.58-0.95]).
Conclusions: Consistent and significant decreases in both overall and VT IPD in children occurred quickly and were sustained for 7 years after PCV7 introduction, supporting use of PCVs. Increases in NVT IPD occurred in most sites, with variable magnitude. These findings may not represent the experience in low-income countries or the effects after introduction of higher valency PCVs. High-quality, population-based surveillance of serotype-specific IPD rates is needed to monitor vaccine impact as more countries, including low-income countries, introduce PCVs and as higher valency PCVs are used. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The manuscript coauthors and members of the Serotype Replacement Study Group have the following conflicts: RD has, in the last five years, received grants/research support from Berna/Crucell, Wyeth/Pfizer, MSD and Protea; he has been a scientific consultant for Berna/Crucell, GlaxoSmithKline, Norvatis, Wyeth/Pfizer, Protea, MSD; he has been a speaker for Berna/Crucell, GlaxoSmithKline, and Wyeth/Pfizer; he is a shareholder of Protea/NASVAX. PDW has received research grants, honoraria, and travel expense reimbursements from vaccine manufacturers including Glaxo SmithKline, Norvatis, Sanofi Pasteur, Merck, and Wyeth, as well as from governmental agencies including the Quebec Ministry of Health and Social Services, Health Canada, and the Public Health Agency of Canada. JE has served as a member of a data safety monitoring board (DSMB) for Novartis meningococcal and typhoid vaccines and participated in an advisory meeting of their pneumococcal protein vaccine in 2009. JE works at the National Institute for Health and Welfare (THL), Helsinki, Finland, which has a research contract with GSK on pneumococcal vaccines. MH is a lead investigator for the Switzerland IPD surveillance program, which is partly funded by an unrestricted grant from Pfizer. JDK and OV are lead investigators of the Calgary
Figures












References
-
- World Health Organization (2012) Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. Available: http://www.who.int/immunization_monitoring/burden/Pneumo_hib_estimates/e.... Accessed 18 February 2013.
-
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, et al. (2010) Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201: 32–41. - PubMed
-
- Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, et al. (2009) Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis 49: 205–212. - PubMed
-
- Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, et al. (2007) Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297: 1784–1792. - PubMed
-
- O’Brien KL, Millar EV, Zell ER, Bronsdon M, Weatherholtz R, et al. (2007) Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 196: 1211–1220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

