Nasopharyngeal carriage of pneumococci four years after community-wide vaccination with PCV-7 in The Gambia: long-term evaluation of a cluster randomized trial
- PMID: 24086259
- PMCID: PMC3785494
- DOI: 10.1371/journal.pone.0072198
Nasopharyngeal carriage of pneumococci four years after community-wide vaccination with PCV-7 in The Gambia: long-term evaluation of a cluster randomized trial
Abstract
Background: A village-randomized trial of a seven-valent pneumococcal-conjugate-vaccine (PCV-7) conducted in rural Gambia showed a decrease of vaccine-type (VT) and a non-significant increase in non-vaccine-type (NVT) nasopharyngeal carriage of pneumococci two years after vaccination. Here, we report findings four years after vaccination.
Methods: PCV-7 was given to all children below 30 months of age enrolled in the trial and to those born during its course in all study villages. Villages were randomized (older children and adults) to receive PCV-7 (wholly vaccinated villages) or serogroup-C-meningococcal-conjugate-vaccine (partly vaccinated villages). Cross-sectional surveys (CSS) to collect nasopharyngeal swabs were conducted before and at various intervals after vaccination. Sixteen of these randomized villages (8 wholly vaccinated and 8 partly vaccinated) participated in a CSS conducted four years after vaccination started.
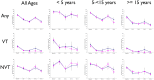
Results: Four years after vaccination, the prevalence of VT pneumococcal carriage was slightly higher in partly than in wholly vaccinated villages [6.4% versus 3.9% (p = 0.120)] compared to 24.4% in the pre-vaccination CSS (p<0.001). Prevalence of NVT four years after vaccination was similar between study groups [32.7% versus 29.8% (p = 0.392), respectively] compared to 51.1% in the pre-vaccination CSS (p<0.001). Four years after vaccination started, lower prevalence of serotype 6A was detected in wholly vaccinated than in partly vaccinated villages (1.6% versus 3.5%, p = 0.093) whilst the prevalence of serotype 19A was similar between groups (2.9% versus 2.5%, p = 0.779). The most prevalent serotype 19A clone was ST 847. The most prevalent serotype 6A clone before vaccination was ST3324 whilst after vaccination ST913 and ST1737 predominated. Fourteen out of 26 STs detected among the serotype 6A isolates were new while no new 19A serotype ST was found.
Conclusions: The decline in prevalence of VT pneumococci seen shortly after PCV-7 vaccination was sustained four years later with only a small difference between study arms. No significant serotype replacement was detected.
Trial registration: ClinicalTrials.gov ISRCTN51695599.
Conflict of interest statement
Figures


References
-
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, et al. (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374: 893–902 S0140-6736(09)61204-6 [pii];10.1016/S0140-6736(09)61204-6 [doi] - DOI - PubMed
-
- Cheung YB, Zaman SM, Nsekpong ED, Van Beneden CA, Adegbola RA, et al. (2009) Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediatr Infect Dis J 28: 990–995 10.1097/INF.0b013e3181a78185 [doi] - DOI - PubMed
-
- Dagan R, Givon-Lavi N, Zamir O, Sikuler-Cohen M, Guy L, et al. (2002) Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis 185: 927–936 JID010101 [pii];10.1086/339525 [doi] - DOI - PubMed
-
- Hill PC, Townend J, Antonio M, Akisanya B, Ebruke C, et al. (2010) Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis 50: 1468–1476 10.1086/652443 [doi] - DOI - PubMed
-
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, et al. (2005) Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294: 2043–2051 294/16/2043 [pii];10.1001/jama.294.16.2043 [doi] - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

