Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease
- PMID: 24086266
- PMCID: PMC3781134
- DOI: 10.1371/journal.pone.0072904
Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease
Abstract
Background and aim: Alcohol-related liver disease (ALD) remains a leading cause of liver-related morbidity and mortality. Age, fibrosis stage, MELD score and continued alcohol consumption predict outcome in everyday clinical practice. In previous studies increased hepatocyte nuclear area and hepatocyte expression of p21, both markers of senescence, were associated with increased fibrosis stage and a poor outcome in non-alcohol-related fatty liver disease, while increased hepatocyte nuclear area was related to liver dysfunction in ALD cirrhosis. This study, therefore, investigated the pattern of hepatocyte cell cycle phase distribution and hepatocyte p21 expression in relation to outcome in ALD.
Methods: Liver sections from two cohorts were studied. The first comprised 42 patients across the full spectrum of ALD. The second cohort comprised 77 patients with ALD cirrhosis. Immunohistochemistry assessed hepatocyte expression of cell cycle phase markers and p21. Regenerating liver (n=12) and "normal" liver sections (n=5) served as positive and negative controls, respectively.
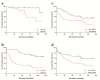
Results: In the first cohort there was little cell cycle progression beyond G1/S phase and increased hepatocyte p21 expression (p<0.0001), which correlated independently with fibrosis stage (p=0.005) and an adverse liver-related outcome (p=0.03). In the second cohort, both hepatocyte p21 expression (p<0.001) and MELD score (p=0.006) were associated independently with an adverse liver-related outcome; this association was stronger with hepatocyte p21 expression (AUROC 0.74; p=0.0002) than with MELD score (AUROC 0.59; p=0.13). Further, hepatocyte p21 expression co-localised with increased hepatic stellate cell activation.
Conclusions: The findings are consistent with impaired cell cycle progression beyond the G1/S phase in ALD. The striking independent associations between increased hepatocyte p21 expression and both fibrosis stage and an adverse liver-related outcome in both cohorts suggests hepatocyte senescence plays an important role in ALD. Measuring hepatocyte p21 expression is simple and cheap and in this series was a useful measure of long-term prognosis in ALD.
Conflict of interest statement
Figures




References
-
- Gao B, Bataller R (2011) Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572-1585. doi:10.1053/j.gastro.2011.09.002. PubMed: 21920463. - DOI - PMC - PubMed
-
- O’Shea RS, Dasarathy S, McCullough AJ (2010) Alcoholic liver disease. Hepatology 51: 307-328. doi:10.1002/hep.23258. PubMed: 20034030. - DOI - PubMed
-
- European Association For The Study Of The L (2012) EASL Clinical Practical Guidelines: Management of alcoholic Liver Disease. J Hepatol 57: 399-420. - PubMed
-
- Dey A, Cederbaum AI (2006) Alcohol and oxidative liver injury. Hepatology 43: S63-S74. doi:10.1002/hep.20957. PubMed: 16447273. - DOI - PubMed
-
- Lucey MR, Mathurin P, Morgan TR (2009) Alcoholic hepatitis. N Engl J Med 360: 2758-2769. doi:10.1056/NEJMra0805786. PubMed: 19553649. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

