Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial
- PMID: 24086607
- PMCID: PMC3784573
- DOI: 10.1371/journal.pone.0075665
Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial
Abstract
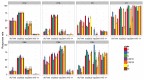
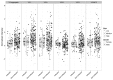
Neutralizing and non-neutralizing antibodies to linear epitopes on HIV-1 envelope glycoproteins have potential to mediate antiviral effector functions that could be beneficial to vaccine-induced protection. Here, plasma IgG responses were assessed in three HIV-1 gp120 vaccine efficacy trials (RV144, Vax003, Vax004) and in HIV-1-infected individuals by using arrays of overlapping peptides spanning the entire consensus gp160 of all major genetic subtypes and circulating recombinant forms (CRFs) of the virus. In RV144, where 31.2% efficacy against HIV-1 infection was seen, dominant responses targeted the C1, V2, V3 and C5 regions of gp120. An analysis of RV144 case-control samples showed that IgG to V2 CRF01_AE significantly inversely correlated with infection risk (OR= 0.54, p=0.0042), as did the response to other V2 subtypes (OR=0.60-0.63, p=0.016-0.025). The response to V3 CRF01_AE also inversely correlated with infection risk but only in vaccine recipients who had lower levels of other antibodies, especially Env-specific plasma IgA (OR=0.49, p=0.007) and neutralizing antibodies (OR=0.5, p=0.008). Responses to C1 and C5 showed no significant correlation with infection risk. In Vax003 and Vax004, where no significant protection was seen, serum IgG responses targeted the same epitopes as in RV144 with the exception of an additional C1 reactivity in Vax003 and infrequent V2 reactivity in Vax004. In HIV-1 infected subjects, dominant responses targeted the V3 and C5 regions of gp120, as well as the immunodominant domain, heptad repeat 1 (HR-1) and membrane proximal external region (MPER) of gp41. These results highlight the presence of several dominant linear B cell epitopes on the HIV-1 envelope glycoproteins. They also generate the hypothesis that IgG to linear epitopes in the V2 and V3 regions of gp120 are part of a complex interplay of immune responses that contributed to protection in RV144.
Conflict of interest statement
Figures



References
-
- Mascola JR, Montefiori DC (2010) The role of antibodies in HIV vaccines. Annu Rev Immunol 28: 413-444. doi:10.1146/annurev-immunol-030409-101256. PubMed: 20192810. - DOI - PubMed
-
- Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R et al. (1999) Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 5: 204–210. doi:10.1038/5568. PubMed: 9930869. - DOI - PubMed
-
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB et al. (2000) Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6: 207–210. doi:10.1038/72318. PubMed: 10655111. - DOI - PubMed
-
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM et al. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15: 951-955. doi:10.1038/nm.1974. PubMed: 19525965. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

