Induction of cytopathogenicity in human glioblastoma cells by chikungunya virus
- PMID: 24086645
- PMCID: PMC3783433
- DOI: 10.1371/journal.pone.0075854
Induction of cytopathogenicity in human glioblastoma cells by chikungunya virus
Abstract
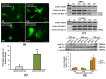
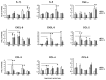
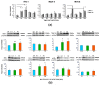
Chikungunya virus (CHIKV), an arthritogenic old-world alphavirus, has been implicated in the central nervous system (CNS) infection in infants and elderly patients. Astrocytes are the major immune cells of the brain parenchyma that mediate inflammation. In the present study we found that a local isolate of CHIKV infect and activate U-87 MG cells, a glioblastoma cell line of human astrocyte origin. The infection kinetics were similar in infected U-87 MG cells and the human embryo kidney (HEK293) cells as indicated by immunofluorescence and plaque assays, 24h post-infection (p.i.). In infected U-87 MG cells, apoptosis was detectable from 48h p.i. evidenced by DNA fragmentation, PARP cleavage, loss of mitochondrial membrane potential, nuclear condensation and visible cytopathic effects in a dose and time-dependent manner. XBP1 mRNA splicing and eIF2α phosphorylation studies indicated the occurrence of endoplasmic reticulum stress in infected cells. In U-87 MG cells stably expressing a green fluorescent protein-tagged light chain-3 (GFP-LC3) protein, CHIKV infection showed increased autophagy response. The infection led to an enhanced expression of the mRNA transcripts of the pro-inflammatory cytokines IL-1β, TNF-α, IL-6 and CXCL9 within 24h p.i. Significant up-regulation of the proteins of RIG-I like receptor (RLR) pathway, such as RIG-I and TRAF-6, was observed indicating the activation of the cytoplasmic-cellular innate immune response. The overall results show that the U-87 MG cell line is a potential in vitro model for in depth study of these molecular pathways in response to CHIKV infection. The responses in these cells of CNS origin, which are inherently defective in Type I interferon response, could be analogous to that occurring in infants and very old patients who also have a compromised interferon-response. The results also point to the intriguing possibility of using this virus for studies to develop oncolytic virus therapy approaches against glioblastoma, a highly aggressive malignancy.
Conflict of interest statement
Figures


References
-
- Das T, Jaffar-Bandjee MC, Hoarau JJ, Krejbich Trotot P, Denizot M et al. (2010) Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog Neurobiol 91: 121-129. doi:10.1016/j.pneurobio.2009.12.006. PubMed: 20026374. - DOI - PubMed
-
- Arpino C, Curatolo P, Rezza G (2009) Chikungunya and the nervous system: what we do and do not know. Rev Med Virol 19: 121-129. doi:10.1002/rmv.606. PubMed: 19274635. - DOI - PubMed
-
- Lewthwaite P, Vasanthapuram R, Osborne JC, Begum A, Plank JL et al. (2009) Chikungunya virus and central nervous system infections in children, India. Emerg Infect Dis 15: 329-331. doi:10.3201/eid1502.080902. PubMed: 19193287. - DOI - PMC - PubMed
-
- Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS et al. (2009) Neurological complications of Chikungunya virus infection. Neurol India 57: 177-180. doi:10.4103/0028-3886.51289. PubMed: 19439849. - DOI - PubMed
-
- Griffin DE (2010) Emergence and re-emergence of viral diseases of the central nervous system. Prog Neurobiol 91: 95-101. doi:10.1016/j.pneurobio.2009.12.003. PubMed: 20004230. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

