Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14
- PMID: 24086697
- PMCID: PMC3785436
- DOI: 10.1371/journal.pone.0076106
Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14
Abstract
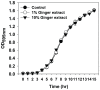
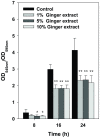
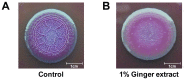
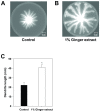
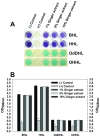
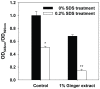
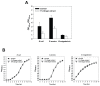
Bacterial biofilm formation can cause serious problems in clinical and industrial settings, which drives the development or screening of biofilm inhibitors. Some biofilm inhibitors have been screened from natural products or modified from natural compounds. Ginger has been used as a medicinal herb to treat infectious diseases for thousands of years, which leads to the hypothesis that it may contain chemicals inhibiting biofilm formation. To test this hypothesis, we evaluated ginger's ability to inhibit Pseudomonas aeruginosa PA14 biofilm formation. A static biofilm assay demonstrated that biofilm development was reduced by 39-56% when ginger extract was added to the culture. In addition, various phenotypes were altered after ginger addition of PA14. Ginger extract decreased production of extracellular polymeric substances. This finding was confirmed by chemical analysis and confocal laser scanning microscopy. Furthermore, ginger extract formed noticeably less rugose colonies on agar plates containing Congo red and facilitated swarming motility on soft agar plates. The inhibition of biofilm formation and the altered phenotypes appear to be linked to a reduced level of a second messenger, bis-(3'-5')-cyclic dimeric guanosine monophosphate. Importantly, ginger extract inhibited biofilm formation in both Gram-positive and Gram-negative bacteria. Also, surface biofilm cells formed with ginger extract detached more easily with surfactant than did those without ginger extract. Taken together, these findings provide a foundation for the possible discovery of a broad spectrum biofilm inhibitor.
Conflict of interest statement
Figures



References
-
- Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2: 95-108. doi:10.1038/nrmicro821. PubMed: 15040259. - DOI - PubMed
-
- Mayer C, Moritz R, Kirschner C, Borchard W, Maibaum R et al. (1999) The role of intermolecular interactions: studies on model systems for bacterial biofilms. Int J Biol Macromol 26: 3-16. doi:10.1016/S0141-8130(99)00057-4. PubMed: 10520951. - DOI - PubMed
-
- Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the "house of biofilm cells". J Bacteriol 189: 7945-7947. doi:10.1128/JB.00858-07. PubMed: 17675377. - DOI - PMC - PubMed
-
- Prosser BL, Taylor D, Dix BA, Cleeland R (1987) Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob Agents Chemother 31: 1502-1506. doi:10.1128/AAC.31.10.1502. PubMed: 3435100. - DOI - PMC - PubMed
-
- Nickel JC, Ruseska I, Wright JB, Costerton JW (1985) Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27: 619-624. doi:10.1128/AAC.27.4.619. PubMed: 3923925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

